Ch. 2: Atoms, Ions, and Molecules
1/123
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
124 Terms
Radioactivity:
the spontaneous emission of high-energy radiation and particles
Do elements in the same column share (periodic table)
similar chemical properties
Why did Thomson’s discovery of the electron lead others to propose that a positive particle might exist within the atom?
Matter is neutral, so some positive particle must be present to provide electrical neutrality
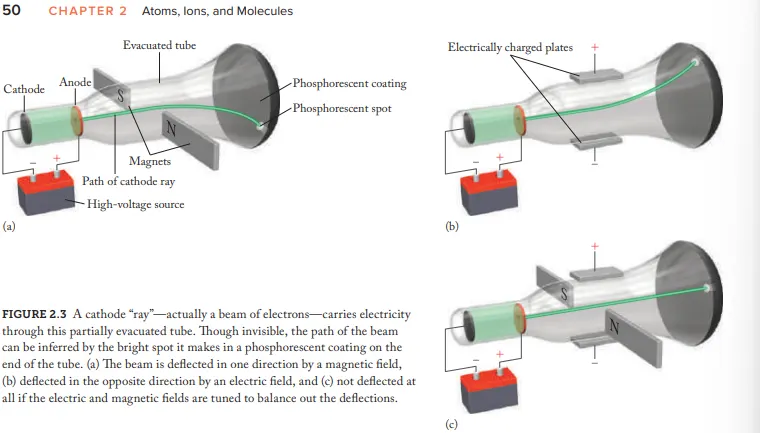
Who created the Cathode Ray Tube (CRT)
Joseph John Thomson
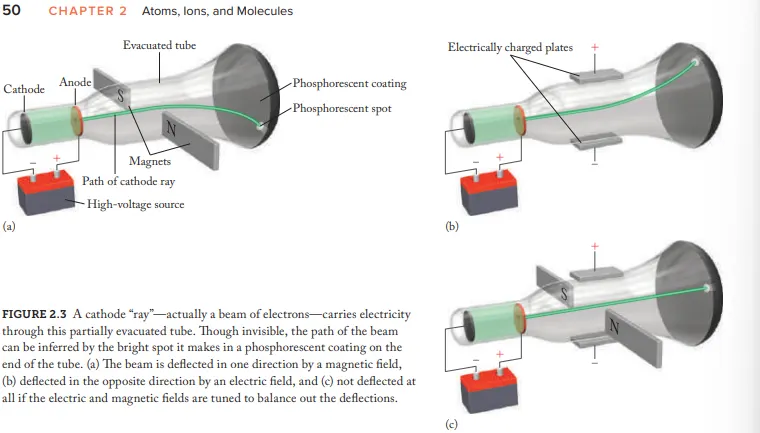
after electricity passes through a CRT what does it transform into
Cathode rays
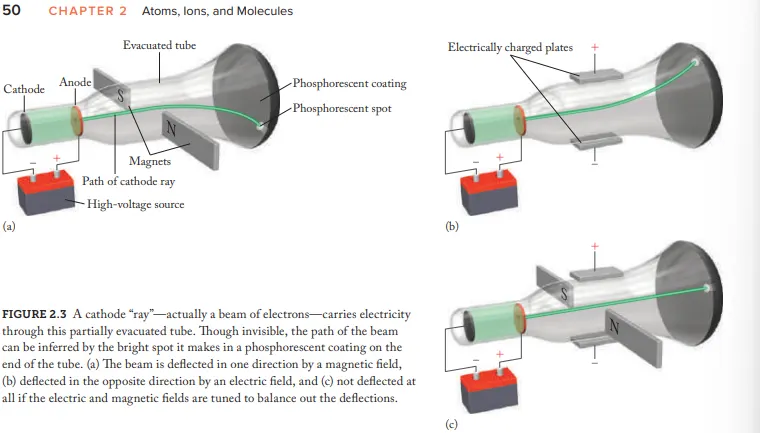
Due to Cathode rays being invisible how where they made visible
coat the opp. end of CRT with phosphorescent material = glow (after beam hits)
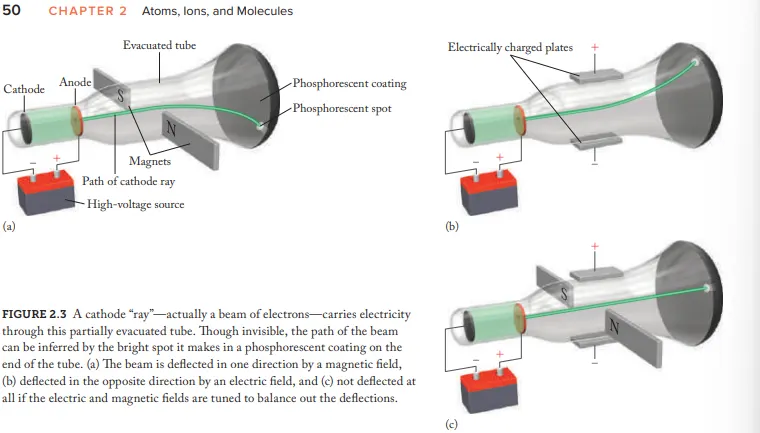
What evidence (using the CRT) is given for the idea that cathode rays aren't energy but negatively charged particles (electrons)
Cathode-ray beams are deflected by magnetic fields and electric fields
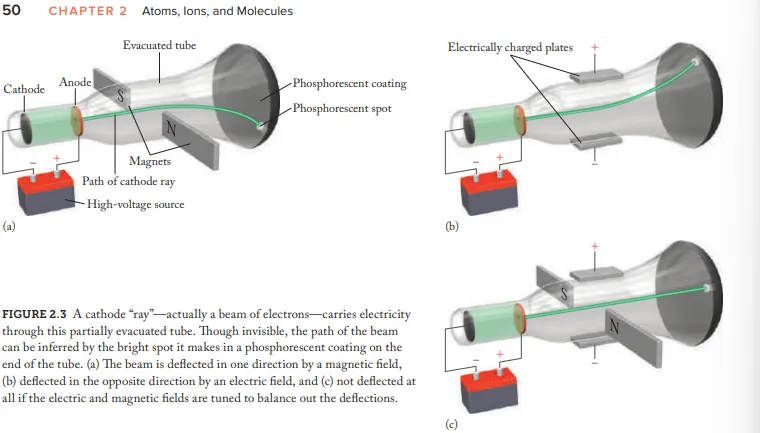
How where the cathode rays balanced
Adjusting the strength of electric/magnetic fields
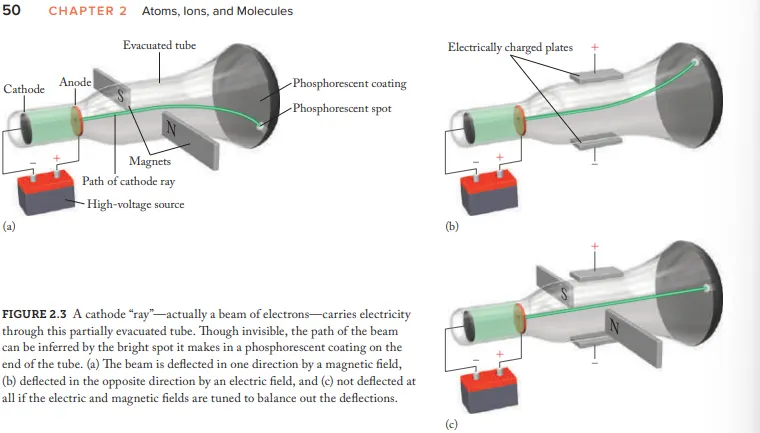
What evidence is there for electrons being fundamental particles present in all forms of matter
Particles always behaved the same way
Particles always had the same mass to charge ratio
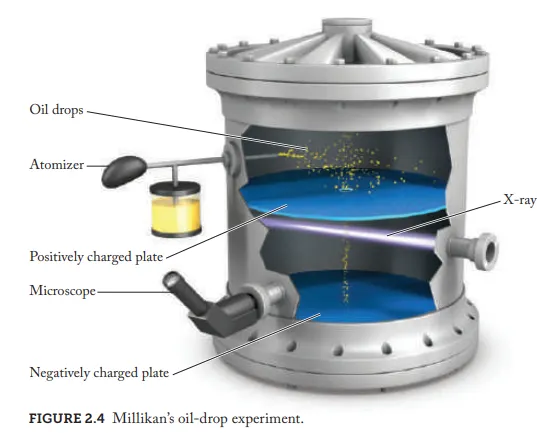
Based on the oil drop experiment what percentage was the concluded charge of a electron out of the modern value
1%
What is the modern measured value of an electrons charge
1.602 x 10^{-19} C
What is the SI unit for energy
C = coulomb
Mass of electron
9.109 x 10^{-28}g
How did Rutherford figure out that protons where part of the nucleus
Rutherford: Bombarding elements with (𝛂)particles = change(transmute)
Hydrogen nuclei sometimes produced (transmutation Rxn)
Conclusion
Hydrogen Nuclei = proton = part of all nuclei
What charge do neutrons have
neutral particles
What’s the symbol for a neutron
n^0
Neutron (in atomic mass units (u))
1.00867 = 1
What’s the symbol for a proton
p^1
What’s the symbol for a electron
e^-1
Proton (in atomic mass units (u))
1.00728 = 1
Electron (in atomic mass units (u))
5.485799 × 10^-4 = 0
Neutron (in grams (g))
1.67493 x 10^-24
Proton (in grams (g))
1.67262 = 10^-24
Electrons (in grams (g))
9.10939 = 10^-28
Neutron (relative value)
0
Proton (relative value)
1+
Electron (relative value)
1-
Neutron (change (Celsius))
0
Proton (change (Celsius)(Charge))
+1.602 = 10^-19
Electron (change (Celsius)(Charge))
-1.602 = 10^-19
Units of mass for Subatomic particles
unified atomic mass units(u) or Daltons (Da)
In terms of carbon what does 1 u equal
1/12 the mass of carbon atom that has six protons and neutrons int its nucleus
In terms of grams what does 1 u equal
1.66054 x 10^{-24}g
State John Dalton’s atomic theory
Each element consists of atoms
atom = indestructible particles
not indestructible
Atoms of an element =
identical
Not quite identical
dif. from atoms of other elements
Atoms = Combine in simple ratios of whole numbers to form compounds
Atoms =
do not(chem. rxn)…
change
can change in nuclear rxns
created
destroyed
rxn → only changes arrangement in which atoms are bond
What was incorrect in John Daltons atomic Theory
Each element consists of atoms
atom = indestructible particles (incorrect)
not indestructible (correct)
Atoms of an element =
identical (incorrect)
Not quite identical (correct)
Atoms =
do not(chem. rxn)…
change (incorrect)
can change in nuclear rxns (correct)
The radioactive atoms measured in the Baby Tooth Survey were strontium-90. Only one of the nuclides below is an isotope of strontium. Which one is it and why?
^87_38 X ^90_40 X ^234_90 X
^87_38X is an isotope of strontium because its atomic number is 38 (each of its nuclei contains 38 protons). ^90_40X is an isotope of zirconium with a mass number of 90 (40 protons, 50 neutrons), and ^234_90X is an isotope of thorium because it has an atomic number of 90.
Write symbols in the form ^A_ZX for the nuclides that have (a) 6 protons and 6 neutrons, (b) 11 protons and 12 neutrons, and (c) 92 protons and 143 neutrons.
A. ^12_6 C
B. ^23_11 Na
C. ^235_92 U
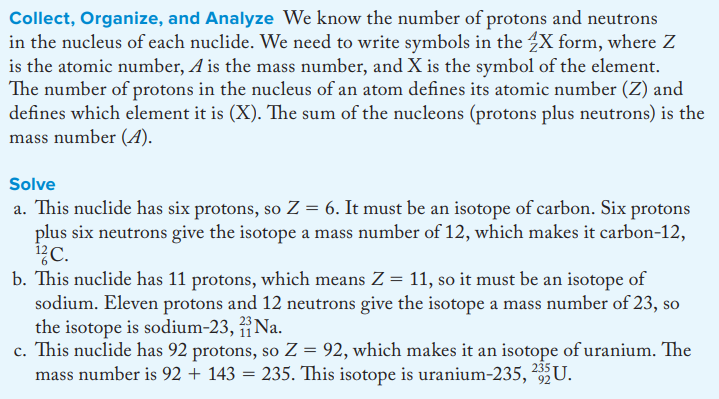
Use the format A^X to write the symbols of the nuclides having (a) 26 protons and 30 neutrons, (b) 7 protons and 8 neutrons, (c) 17 protons and 20 neutrons, and (d) 19 protons and 20 neutrons.
a. 56^Fe
b. 15^N
c. 37^Cl
d. 39^K
How many neutrons are in each of the following nuclides: (a) 14^N; (b) 32^P; (c) 157^Gd?
a. 7
b. 17
c. 93
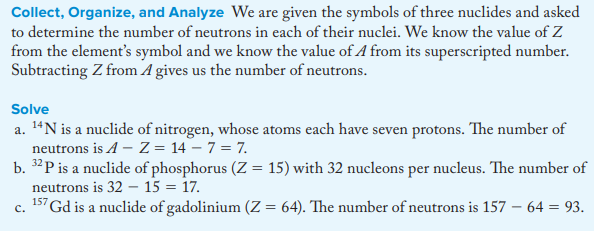
Determine the number of protons and neutrons in each of these radioactive nuclides: (a) 60^Co, used in cancer therapy; (b) 131^I, used in thyroid therapy; (c) 192^Ir, used to treat coronary disease.
.(a) 27 p, 33 n; (b) 53 p, 78 n; (c) 77 p, 115 n
During Francis W. Aston experiment on positively charged particles what was the difference between a cathode ray and a positive ray
Modified cathode ray tubes(mostly evacuated) + fill gases(ex. neon)= conventional beams of cathode rays and 2ndary beams of positively charge particles
Cathode ray: streams of electrons that all have the same mass and charge regardless of the cathode material/fill gas
Positive rays: masses(particles) depend on the identity of the fill gas
Why are positive rays dependent on the identity of the fill gas?
Not individual protons
Rather… atoms of the fill gas that had lost electrons to form positively charge ions
Positive ray analyzer (type A)
A
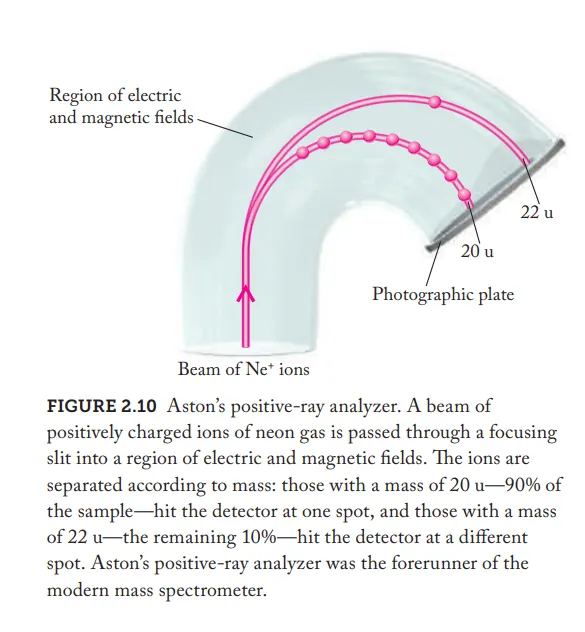
What idea did Francis W. Aston’s experiment(positive rays) contradict?
contradicted the idea that each element was composed of the identical atoms (each have the same mass)
From Francis W. Aston’s experiment(positive rays) what where the resulting findings form the neon atoms
Neon consists two kinds of isotopes
Isotopes of neon
Both:
same number of protons (10)
Iso(1):
10 neutrons
Total mass = 20 u
Iso(2):
12 neutrons
Total mass: 22u
Do atoms of the same element differ in protons or in neutrons
protons
do atoms of the same element differ in mass
yes
what determines the atomic number(Z)
number of protons
what identifies the atom of a element
number of protons
what is the periodic table ordered by?
atomic number
What defines the mass number(A)
Number of Nucleons(in the nucleus)
Nucleon
neutrons and protons
What defines an isotope (in terms of atomic number and mass numbers)
same atomic number(Z) + different mass numbers
What makes a neutral atom?
#electrons = #protons
Nuclide
specific combination of neutrons and protons (atom)
What’s the symbol for a nuclide
^A_Z X
Within a nuclide symbol what does A represent? ^A_Z X
A = # neutrons
Within a nuclide symbol what does Z represent? ^A_Z X
Z = # protons (Often omitted due to X)
Ex. ^A X
Written = neon-20 and neon-22
Within a nuclide symbol what does X represent? ^A_Z X
X = one/two letter for the element
Ex. ^20_10Ne ^22_10Ne
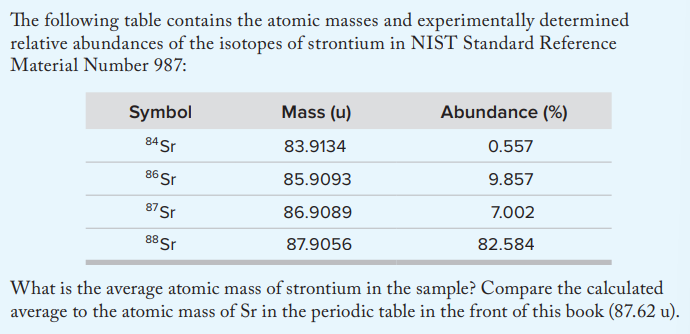
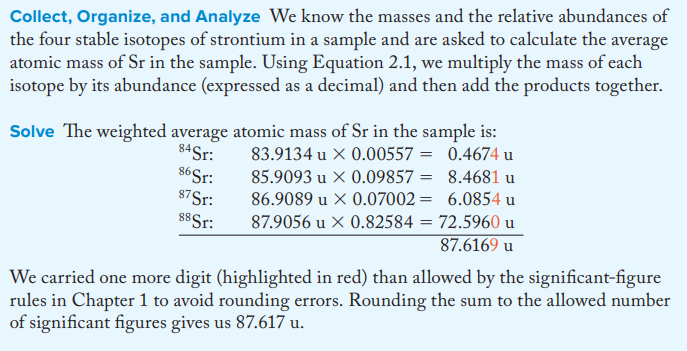
A
87.6169 u
Silver (Ag) has two stable isotopes: silver-107 (106.905 u) and silver-109 (108.905 u). If the average atomic mass of silver is 107.868 u, what is the natural abundance of each isotope? (Hint: Let x be the natural abundance of one of the isotopes. Then 1 2 x is the natural abundance of the other.)
^107Ag = 51.85%; ^109Ag = 48.15%
Atomic wt
weighted average of the masses of all isotopes of the element
Natural Abundances
the relative proportion (percentage or fraction) of each isotope of a chemical element as it is found in nature, before any artificial enrichment or depletion, used to calculate the element's average atomic mass on the periodic tabl
What’s the equation for determining the Atomic wt of an element
mx = a1m1 + a2m2 + a3m3 + …..
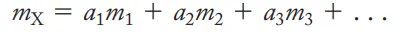
in the following equation(atomic wt) (mx = a1m1 + a2m2 + a3m3 + …..) what does mx represent
average atomic mass of element (X)
in the following equation(atomic wt) (mx = a1m1 + a2m2 + a3m3 + …..) what does m1 represent
masses of isotopes
in the following equation(atomic wt) (mx = a1m1 + a2m2 + a3m3 + …..) what does a1 represent
natural abundances (decimal form)
What causes Isotopic abundances of elements to slightly vary
the origins of samples
the presence of certain other elements in them
Why did Mendeleev skip cells in his periodic table?
Because sometimes the next heaviest element that was known in the early 1870s did not have chemical properties similar to the other elements in the next column to be filled. For example, the next heaviest element after calcium (a group II element in Mendeleev’s table) was titanium, whose properties fit those of the elements in group IV, not group III. So, Mendeleev skipped the cell in group III and placed titanium in group IV. The missing group III element, scandium, was discovered (by a Scandinavian chemist) in 1879.
Use the periodic table on the inside front cover to determine the symbol and name of
each of the following elements:
a. The third-row element in group 14
b. The fourth-row alkaline earth metal
c. The halogen with fewer than 16 protons in its nucleus
(a) Si, silicon; (b) Ca, calcium; (c) F, fluorine.
Write the symbol and name of each element:
a. The metalloid in group 15 closest in mass to the noble gas krypton
b. The element in the fourth row that is an alkali metal
c. The transition metal in the fifth period with chemical properties most like those of zinc (Z = 30)
d. The nonmetal in the fourth period with chemical properties most like those of sulfur
. a. As, arsenic
b. K, potassium
c. Cd, cadmium
d. Se, selenium
Carbon can combine with oxygen to form either CO or CO2 (Figure 2.16), depending on reaction conditions. If 26.6 g of O2 reacts with 10.0 g of C to make CO2, how many grams of O2 react with 10.0 g of C to make CO?
(26.6g O_2) x ½ = 13.3gO_2
Predict the mass of oxygen required to react with 14.0 g of nitrogen to make N2O5 if 16.0 g of oxygen reacts with 14.0 g of nitrogen to make N2O2 (Figure 2.17).
40.0g
Law of Multiple Proportions:
when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios, like 1:2 or 2:3, reflecting the fixed number of atoms combining
what do compounds combine in
ratios
How are the ratios in compounds presented
Chemical formulas
On the topic of ratios what did Dalton state
ratio of different masses of oxygen that react with a given mass of sulfur to form 2 distinct compounds could be expressed as a ratio of two small whole numbers
Law of Multiple Proportions
when two elements form more than one compound, the masses of one element that combine with a fixed mass of the other element are in simple whole-number ratios, like 1:2 or 2:3, reflecting the fixed number of atoms combining
S(10g) + O2O2(10g) = SO2SO2
2S(10g) + 3O2O2(15g) = 2SO3SO3
Ratio of 2 masses of oxygen: 10:15 → 2:3
Compounds composed of pairs of metallic + nonmetallic elements
ionic
Compounds composed of two nonmetals or metalloids
molecular
Molecular Compounds
consist of molecules with two different nonmetals
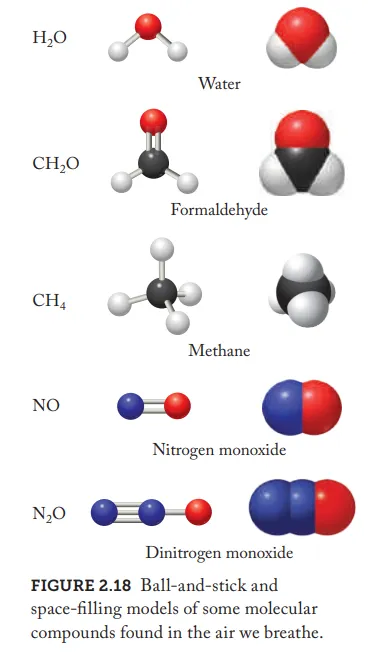
How are molecular compounds bonded
covalent bonds
Covalent bond
a strong chemical link formed when nonmetal atoms share valence electrons to achieve a stable, filled outer shell
Why are there different ways to form covalent bonds between atoms of the same two elements
Due to two elements being able to form compounds with different molecular formulas
Molecular Formulas
shows the exact number and types of atoms in a molecule, using element symbols and subscripts
Which of the following ionic formulas does not represent an electrically neutral compound? (Hint: Base your selection on the charges of the common ions in Figure 2.20.) (a) KBr; (b) CaF2; (c) CsN; (d) TiO2; (e) AgCl
CsN
What is the charge of ionic compounds
neutral
Identify which of the following binary compounds are ionic and which are molecular: (a) sodium bromide (NaBr); (b) carbon dioxide (CO2); (c) lithium iodide (LiI); (d) germanium tetrachloride (GeCl4); (e) calcium chloride (CaCl2)
(a)–(d) are molecular; (e) is ionic.
atoms of metals that have each lost one or more electrons (charge)
+
atoms of nonmetals that have each gained +1 electrons (charge)
negatively charged anions
Empirical Formulas
formulas based on the lowest whole number ratio (describes its formula units)
Ratio of NaCl
1 Na surrounded by 6 Cl
1 Cl surrounded by 6 Na
Ratio: 1:1 (Na:Cl)
What is the only type of formula that can describe a ionic compound
empirical formula
Formula unit:
the smallest electrically neutral unit within the crystal
Monatomic ions
Ions that are each composed of a single atom
Chemical Nomenclature
the systematic naming of chemical compounds using standardized rules
What sphere do organic compounds make up
biosphere
What elements or organic compounds mostly composed of
Composed of mostly carbon + hydrogen + lesser amounts of nitrogen, oxygen, phosphorus, and sulfur
What sphere is composed of inorganic compounds make up?
Geosphere
Geosphere
any of the almost spherical concentric regions of matter that make up the earth and its atmosphere, as the lithosphere and hydrosphere.