Exam 4- Chemistry
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
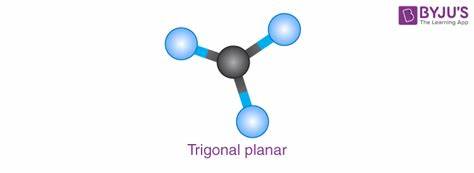
An electron geometry structure is a Trigonal Planar if?
All electron domains are bonding electrons
An electron geometry structure is bent if?
One of the domains is a lone pair
An electron geometry structure is tetrahedral if?
If all are bonding pairs, and contain 4 bonds. No lone pairs.
An electron geometry structure is trigonal pyramidal if?
If there is 1 lone pair ONLY on the central atom
Which of the following bonds has larger electron domains
Double bonds and triple bonds
What happens when double and triple bonds exert a repulsive force?
The bond angles get bigger
What is the electron geometry of electron group 5
Triganol bipyramidal
What is the electron geometry of electron group 6?
Octahedral
What positions are lone pairs placed?
Equatorial positions
What molecular geometry structures belong in group 5
Seesaw, T-shaped, linear, trigonal bipyramidal
What are the molecular geometries included in the group 6 electron domain.
Octahedral and square planar, Square pyramidal
What is the bond angle of a tetrahedral structure?
109.5 degrees
What is the bond angle of a trigonal planar structure?
120 degrees
What is it called when two atoms begin to share the same space
“Overlap” of orbitals causing a covalent bond to form with the same spins
What is a hypervalent molecule?
The elements that have more than an octet
What are the characteristics of a sigma bond
Head-to-head overlap
The electron density is arranged symmetrically around the internuclear axis
Creates stable connections between atoms
What are the characteristics of a pi bond?
Sideways overlap
Electron density is above or below the internuclear axis
What are localized electrons?
Electrons shared between two atoms with a fixed position .
What are delocalized electrons
Unstable bonded electrons that can change positions/locations at any time
What are some of the characteristics of molecular orbitals?
Max number of 2 electrons per orbital
Electrons in the same box (orbital) have opposite spins
Definite energy of orbital
Visualizes electron density
What are bonding orbitals?
Constructive combos of atomic orbitals
What are antibonding orbitals?
Destructive combos of atomic orbitals.
A nodal plane occurs where electron density = 0
What is paramagnetism?
one or more unpaired electrons in an orbital
What is diamagnetism?
All electrons in the orbital are paired.
Weakly repelled by a magnetic field.
What is the electron geometry for group 2?
Linear
What is the electron geometry of electron group 3
Trigonal Planar
What is molecular geometry (M.G)?
The actual geometry of a molecule that depends on the bond arrangement and the effect of the lone pairs (change based on derivative)
Derivative = A modified version of a basic molecule (comes from a basic shape but just with small changes.)
What is a parent molecule?
Electron geometry & molecular geometry are the same because there are no lone pairs on central atom.