3.1.3.6 - Bond polarity
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
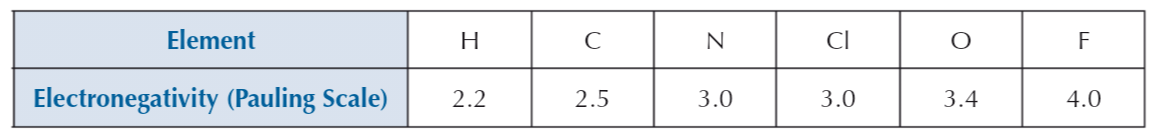
Electronegativity
Atom’s ability to attract electron pair
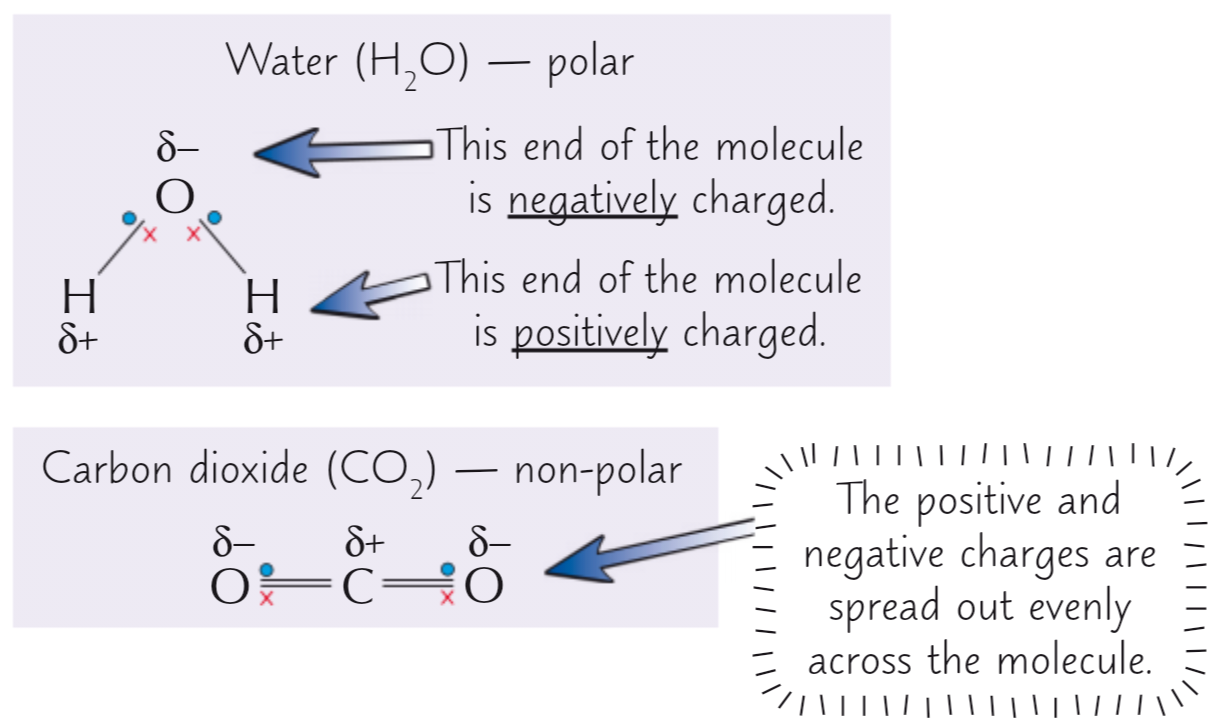
Polar covalent bond forms when ___
Two atoms have different electronegativities
BP of electrons is pulled towards the more electronegative atom
Greater difference in electronegativity = more polar bond
If molecule contains differing electronegativities, distribution of electrons across molecule may be uneven, producing polar covalent bond
Dipole
Difference in charge between two atoms caused by shift in electron density in bond
Permanent dipole
Caused by difference in electronegativity between two atoms in polar bond

Polar molecule
Not all molecules with polar bonds are polar - if polar bonds are arranged symmetrically, charges cancel out + no permanent dipole