Chapter 2 biological molecules
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
Metabolism
The sum total of all the biochemical reactions in the body is known as metabolism.
The four most common elements in living organisms in order of abundance
hydrogen, carbon, oxygen and nitrogen
Why carbon is particularly important than other elements in living organisms?
because carbon atoms can join together to form long chains or ring structures
They can be thought of as the basic skeletons of organic molecules
molecules that contain carbon.(Other atoms, with different functions, are attached to the carbon skeletons)
macromolecule
a large molecule such as a polysaccharide, protein(polypeptides) or nucleic acid(polynucleotides)
polymer
a giant molecule made from many similar repeating subunits joined together in a chain; the subunits are much smaller and simpler molecules known as monomers; examples of biological polymers are polysaccharides, proteins and nucleic acids
monomer
a relatively simple molecule which is used as a basic building block for the synthesis of a polymer; many monomers are joined together by covalent bonds to make the polymer, usually by condensation reactions; common examples of monomers are monosaccharides, amino acids and nucleotides
condensation reaction
a chemical reaction involving the joining together of two molecules by removal of a water molecule
hydrolysis
a chemical reaction in which a chemical bond is broken by the addition of a water molecule; commonly used to break down complex molecules into simpler molecules
monosaccharide
a molecule consisting of a single sugar unit (molecule) ,(‘mono’ means one, ‘saccharide’ means sugar).They have the general formula (CH2O)n
Naturally occurring polymers
Cellulose and rubber
Industrially produced polymers
polyester, polythene, PVC (polyvinyl chloride) and nylon.
Organic polymers
are made up of carbon-based monomers and contain thousands of carbon atoms joined end to end
The general formula for a carbohydrate
C x (H 2 O) y
Three main groups of carbohydrates
monosaccharides, disaccharides and polysaccharides.
The main types of monosaccharides (when classified according to the number of carbon atoms in each molecule)
trioses (3C), pentoses (5C) and hexoses (6C)
Common hexoses
glucose, fructose and galactose
Two common pentoses
ribose and deoxyribose
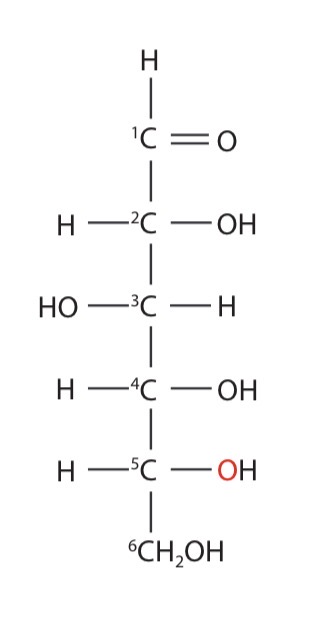
glucose straight-chain form
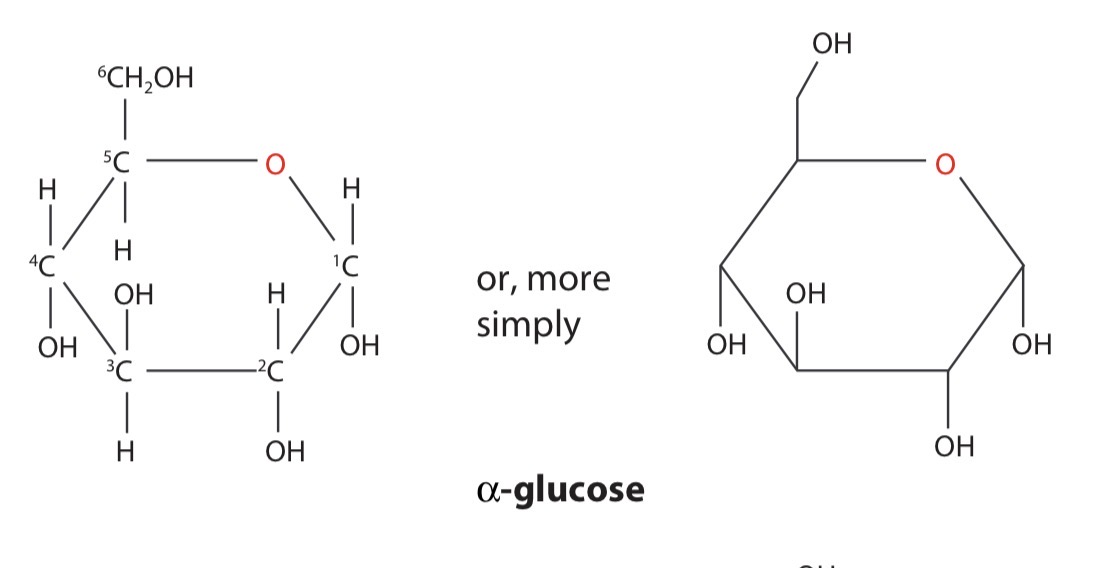
α-glucose
The form of glucose where the hydroxyl group, -OH, on carbon atom 1 is below the ring is known as α-glucose (alpha-glucose)
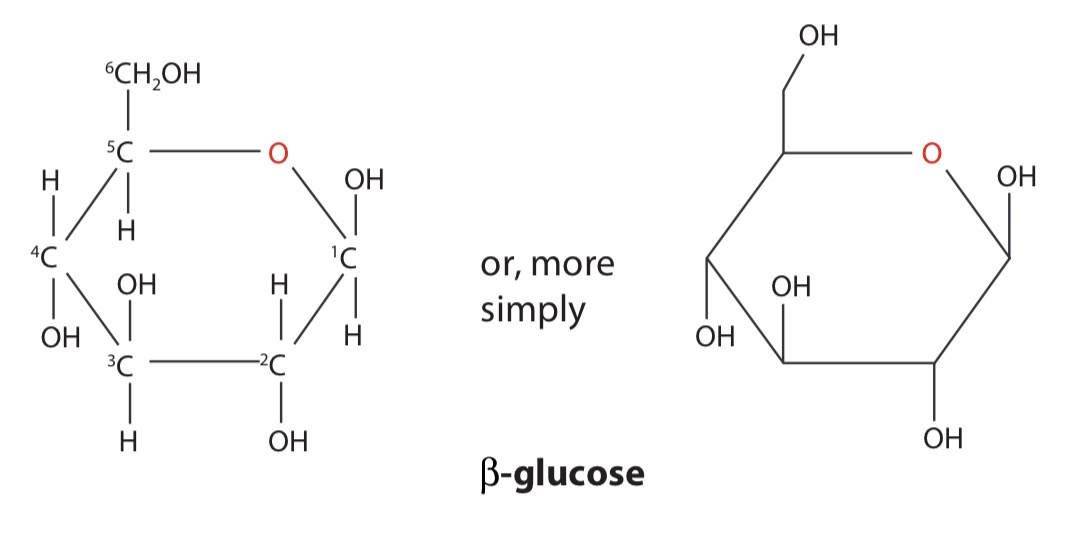
β-glucose
The form of glucose where the hydroxyl group, -OH, on carbon atom 1 is above the ring is known as β-glucose (beta-glucose)
Two major functions of monosaccharides in living organisms
First, they are commonly used as a source of energy in respiration. Second, monosaccharides are important as building blocks for larger molecules.
Why monosaccharides can release a large amount of energy? Which monosaccharide is the most important in energy metabolism?
This is due to the large number of carbonhydrogen bonds. These bonds can be broken to release a lot of energy, which is transferred to help make ATP (adenosine triphosphate) from ADP (adenosine diphosphate) plus phosphate during the process of respiration. The most important monosaccharide in energy metabolism is glucose.
Some examples of the use of monosaccharides as building blocks for larger molecules
For example, glucose is used to make the polysaccharides starch, glycogen and cellulose. Ribose (a pentose) is one of the molecules used to make RNA (ribonucleic acid) and ATP. Deoxyribose (also a pentose) is one of the molecules used to make DNA.
Disaccharide
a sugar molecule consisting of two monosaccharides joined together by a glycosidic bond
The three most common disaccharides
maltose (glucose + glucose), sucrose (glucose + fructose) and lactose (glucose + galactose)
Sucrose
Sucrose is the transport sugar in plants and the sugar commonly bought in shops.
Lactose
Lactose is the sugar found in milk and is therefore an important constituent of the diet of young mammals.
Explain the reaction that forms a glycosidic bond
For each condensation reaction, two hydroxyl (–OH) groups line up alongside each other. One combines with a hydrogen atom from the other to form a water molecule. This allows an oxygen ‘bridge’ to form between the two molecules, holding them together and forming a disaccharide. The bridge is called a glycosidic bond.
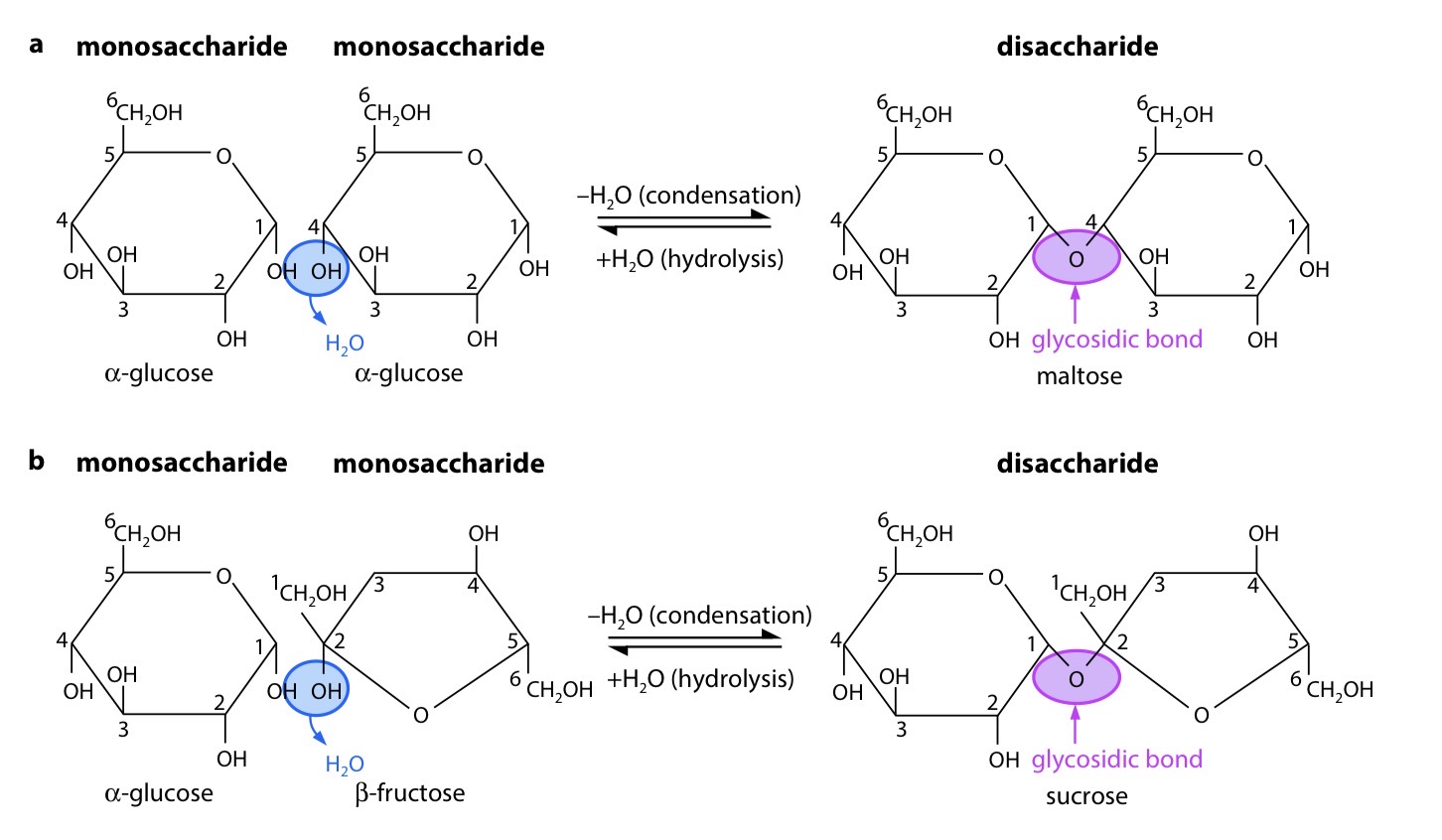
Glycosidic bond
a C – O – C link between two sugar molecules, formed by a condensation reaction; it is a covalent bond
Proteins functions
• all enzymes are proteins
• proteins are essential components of cell membranes
• some hormones are proteins – for example, insulin and glucagon
• the oxygen-carrying pigments haemoglobin and myoglobin are proteins
• antibodies, which attack and destroy invading microorganisms, are proteins
• collagen is a protein that adds strength to many animal tissues – for example, bone and the walls of arteries
• hair, nails and the surface layers of skin contain the protein keratin
• actin and myosin are the proteins responsible for muscle contraction
• proteins may be storage products – for example, casein in milk and ovalbumin in egg white.
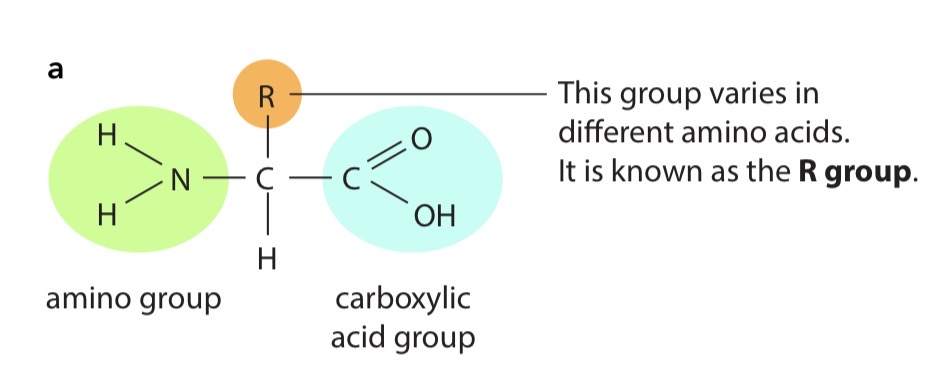
The structure amino acids
All amino acids have a central carbon atom which is bonded to an amino group, –NH 2 , and a carboxylic acid group, –COOH. These two groups give amino acids their name. The third component that is always bonded to the carbon atom is a hydrogen atom. So, the only way in which amino acids differ from each other is in the fourth group of atoms bonded to the central carbon. This is called the R group.
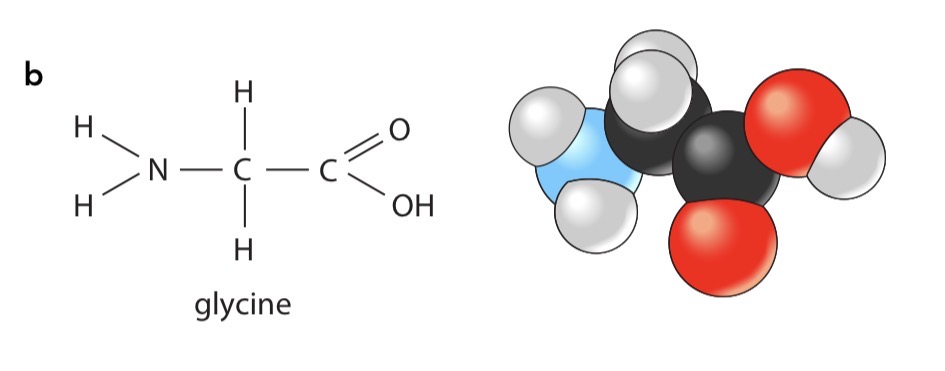
the simplest amino acid
Glycine
Explain the reaction that forms a peptide bond
One loses a hydroxyl (–OH) group from its carboxylic acid group, while the other loses a hydrogen atom from its amino group. This leaves a carbon atom of the first amino acid free to bond with the nitrogen atom of the second. The link is called a peptide bond. The oxygen and two hydrogen atoms removed from the amino acids form a water molecule.
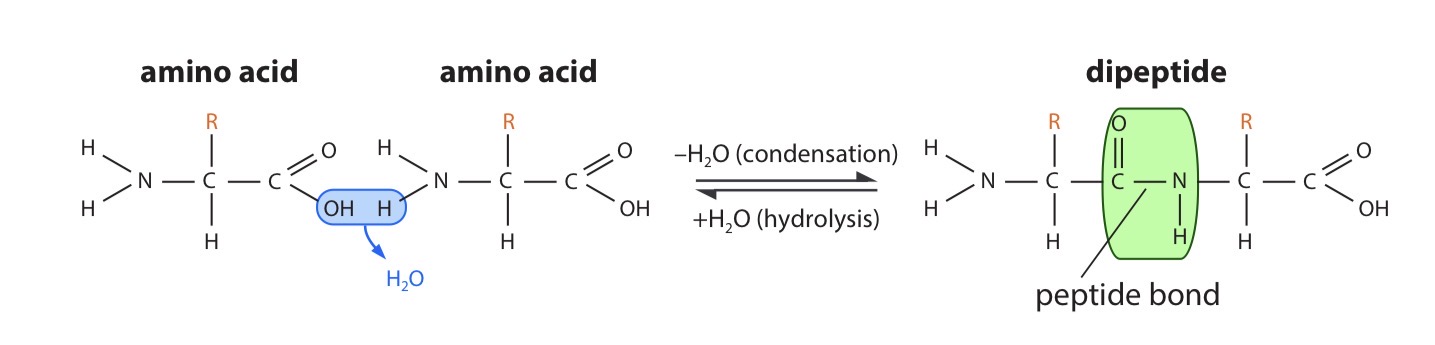
Peptide bond
the covalent bond joining neighbouring amino acids together in proteins; it is a C – N link between two amino acid molecules, formed by a condensation reaction