Structure 1.3 - Electron Configurations (IB 11 Chemistry) | Quizlet
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
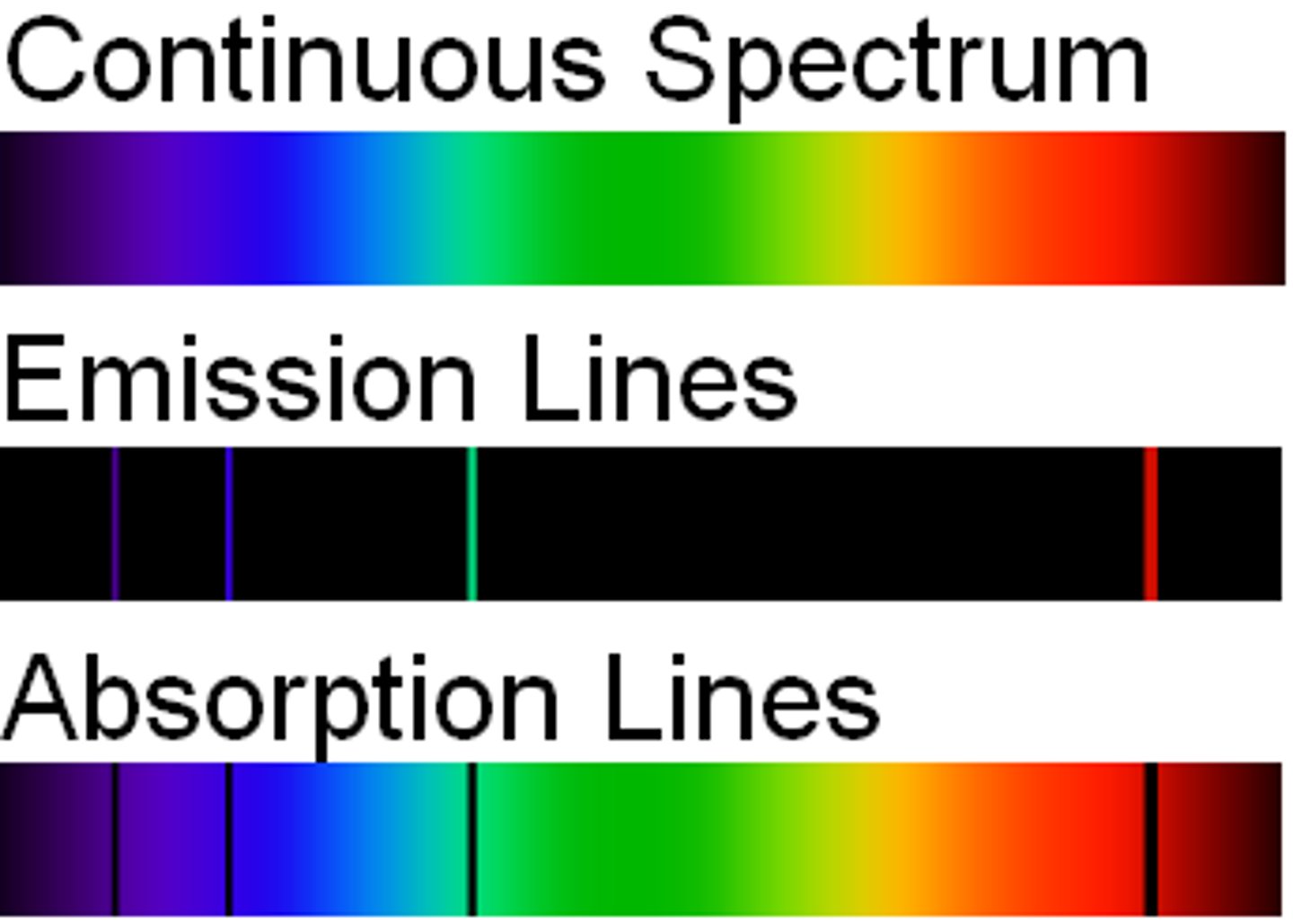
Continuous spectrum
- occurs when white light is passed through a prism
- contains light of all wavelengths
- a series of colours where one colour merges into the next
- no gaps in the spectrum
- order of colours is constant (ROY G BIV)
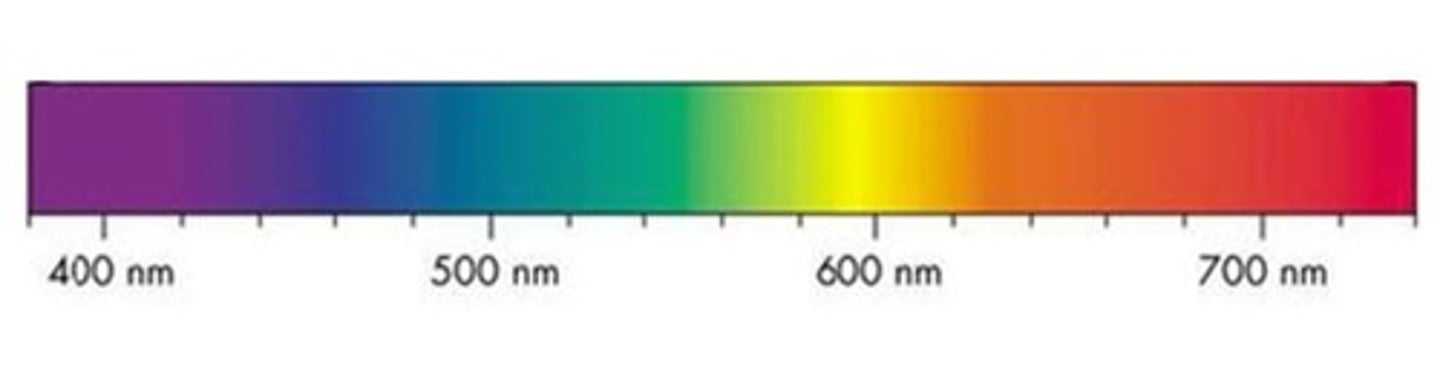
Emission Spectrum
- occurs when the light emitted from a gaseous element under high voltage is passed through a prism
- Produces a series of coloured line against a dark background
- electrons release energy in the form of light as they move back to ground state
Absorption Spectrum
- occurs when white light is passed through a cold gas then through a prism
- Produces a series of black lines on top of a continuous spectrum
- electrons move to higher energy level
- atom in excited state
3 spectra
continuous spectrum, emission spectrum, absorption spectrum
Why the spectra occur
1. electron absorbs energy and jumps to higher level (excited state)
2. Electron stays at higher level for a short period of time
3. energy is released as a photon (light) as the electron returns to lower energy level (ground state)
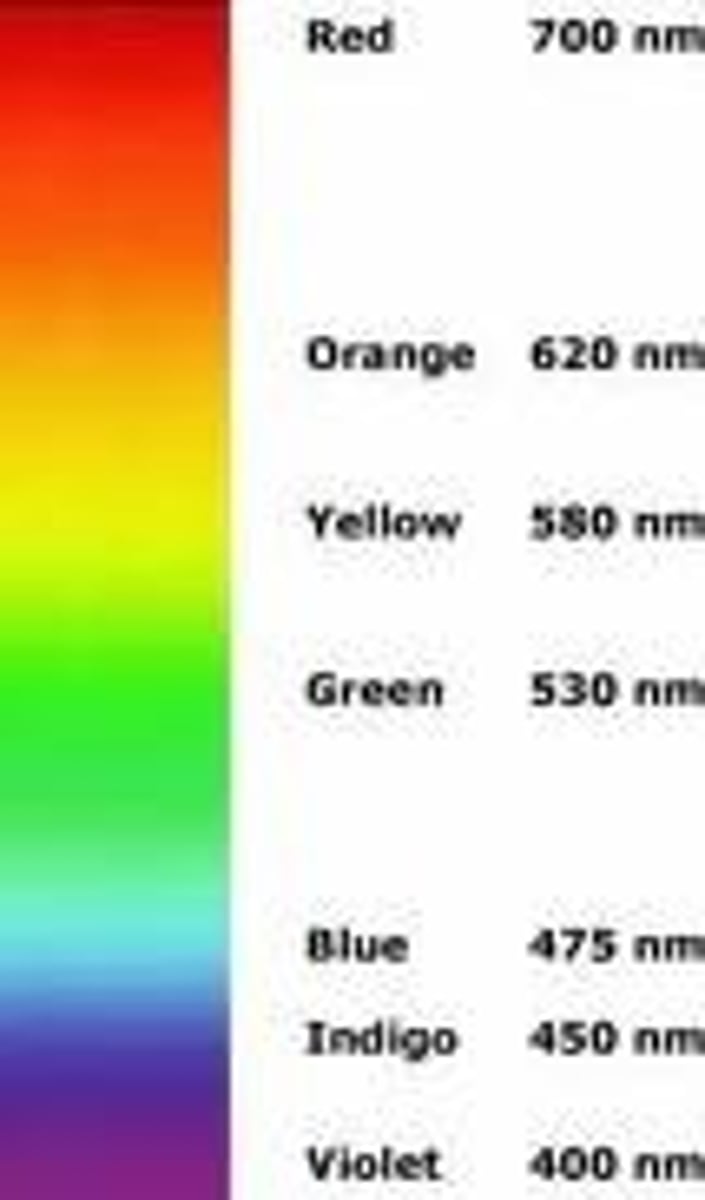
ROY G BIV
Red, orange, yellow, green, blue, indigo, violet
Red has the lowest frequency/energy and longest wavelength
Violet has the highest frequency/energy and lowest wavelength
Ground state
- all electrons in a system are at the lowest energy levels
- "zero" energy
- highly stable
- long lifetime
-electrons lose energy in the form of electromagnetic radiation when returning to ground state
Excited state
- any state of the system that has a higher energy than ground state
- high energy
- highly unstable
- short lifetime
- electrons are located at higher energy level
Photon
a particle of light which essentially is a packet of electromagnetic radiation
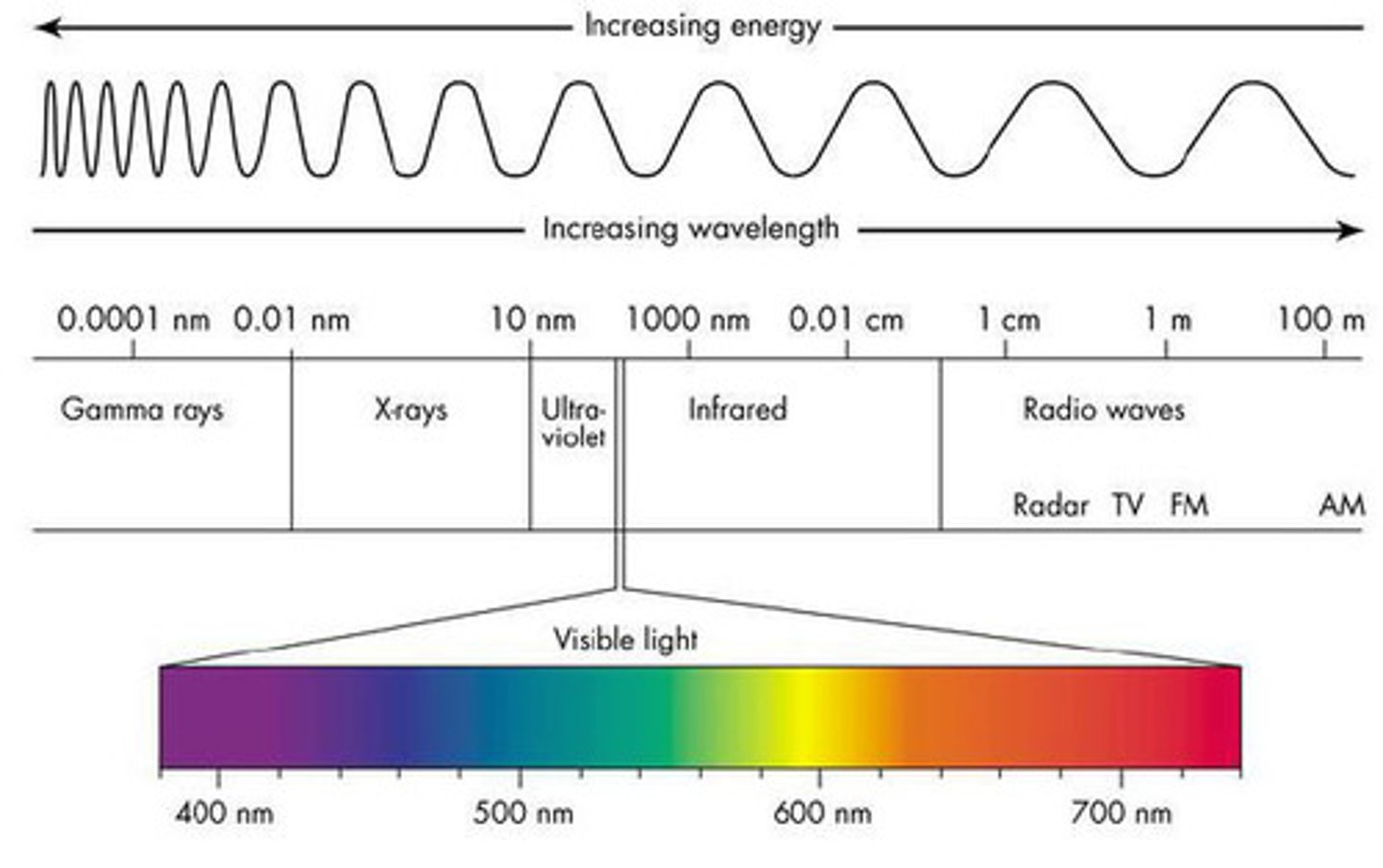
electromagnetic (EM) radiation
A form of energy that exhibits wavelike behavior as it travels through space (and matter if there's enough energy)
Electromagnetic spectrum
All of the frequencies or wavelengths of electromagnetic radiation
Ex. visible light, microwaves, infrared radiation (IR), ultraviolet rays (UV), X-rays, and gamma rays
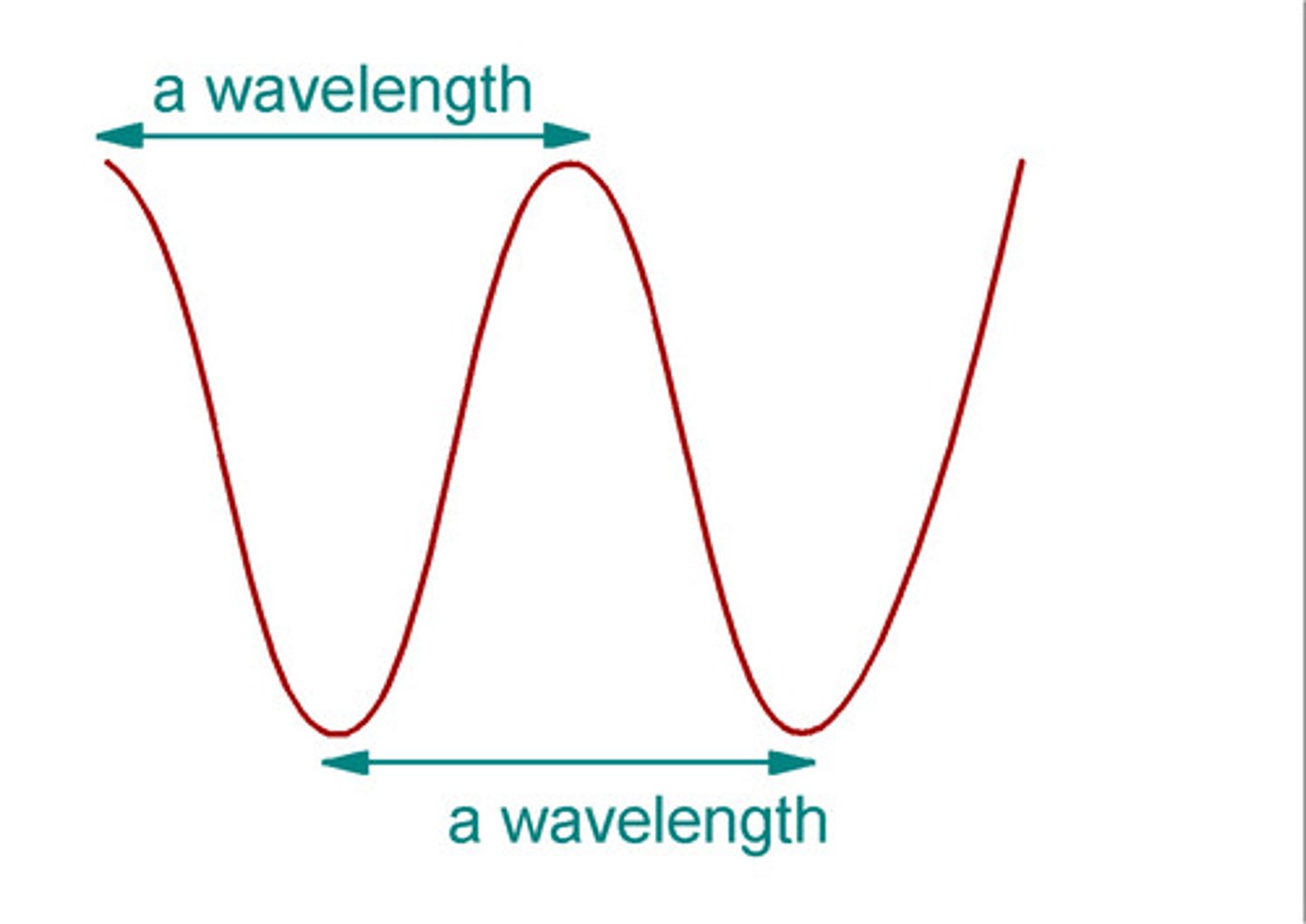
wavelength
- distance between two successive crests in a wave
- symbol is lamba (λ)
- unit is meters (m or nm
Frequency
- # of waves passing through a given point each second
- symbol is f
- unit is hertz (Hz)
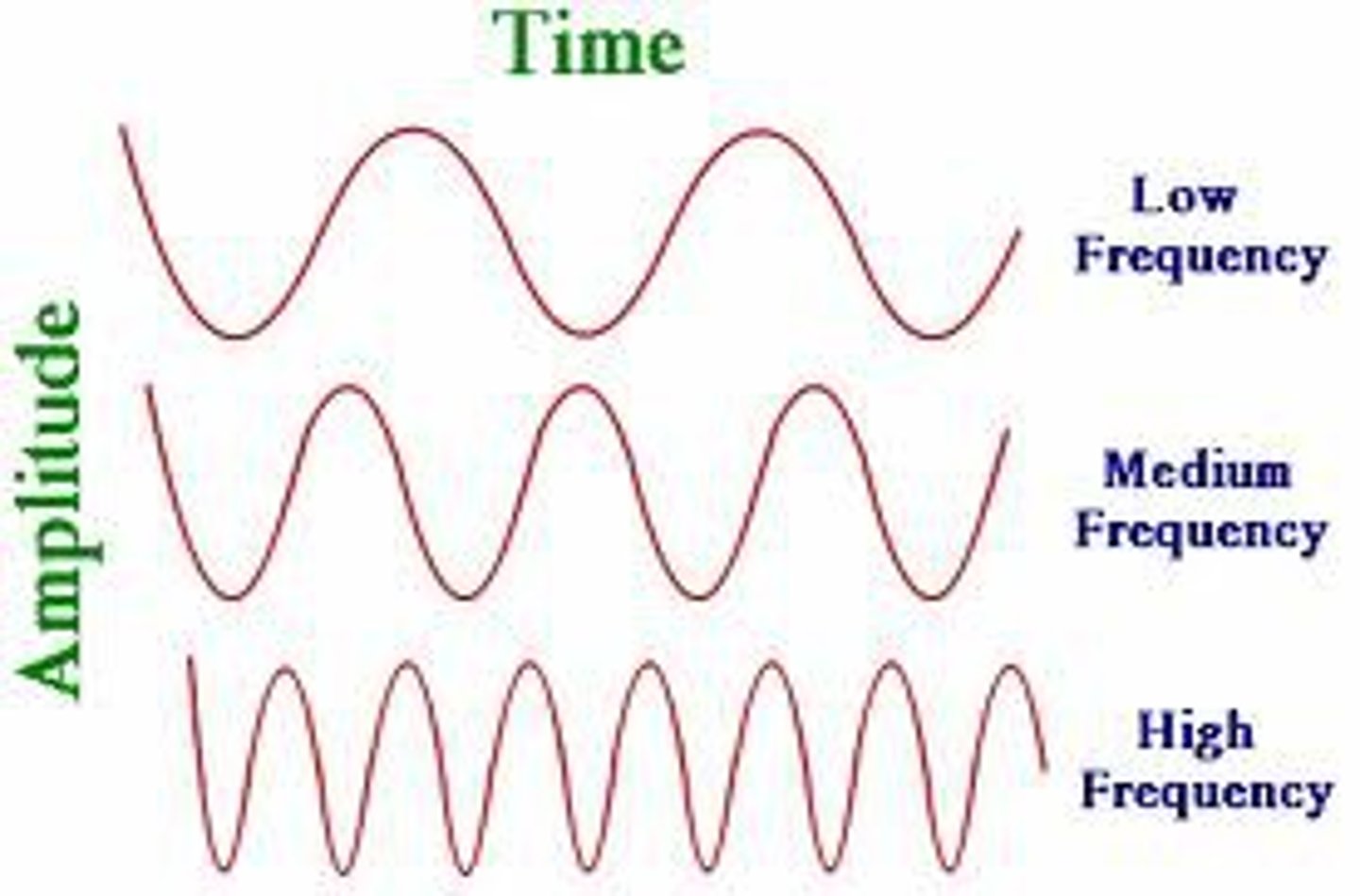
When wavelength increases, frequency and energy ____.
DECREASE
When wavelength decreases, frequency and energy increase
infrared radiation (IR)
- electromagnetic radiation that has long wavelengths and low frequency
- not visible to human eye
- can be used to treat injuries
Ultraviolet
- electromagnetic radiation that has short wavelengths and high frequency
- not visible to human eye
- can cause damage to skin (e.g. the sun)
Quantization
the concept that energy occurs in discrete units called quanta.
Bohr Theory of the Hydrogen Atom
- the electron can only exist in stationary orbits around the nucleus. The orbits are associated with discrete energy levels
- when an electron in the lowest energy level absorbs a photon with the right amount of energy, it will move to a higher energy level for a period of time
- when the electron returns to a lower energy level, it emits a photon of light. The photon represents the energy difference between the two levels
Electrons ____ exist between energy levels
CANNOT
electrons jumping up a level requires a specific amount of energy, and jumping down a level will release the same amount of energy.
Note that electrons will only absorb or release the exact amount of energy to move between levels.
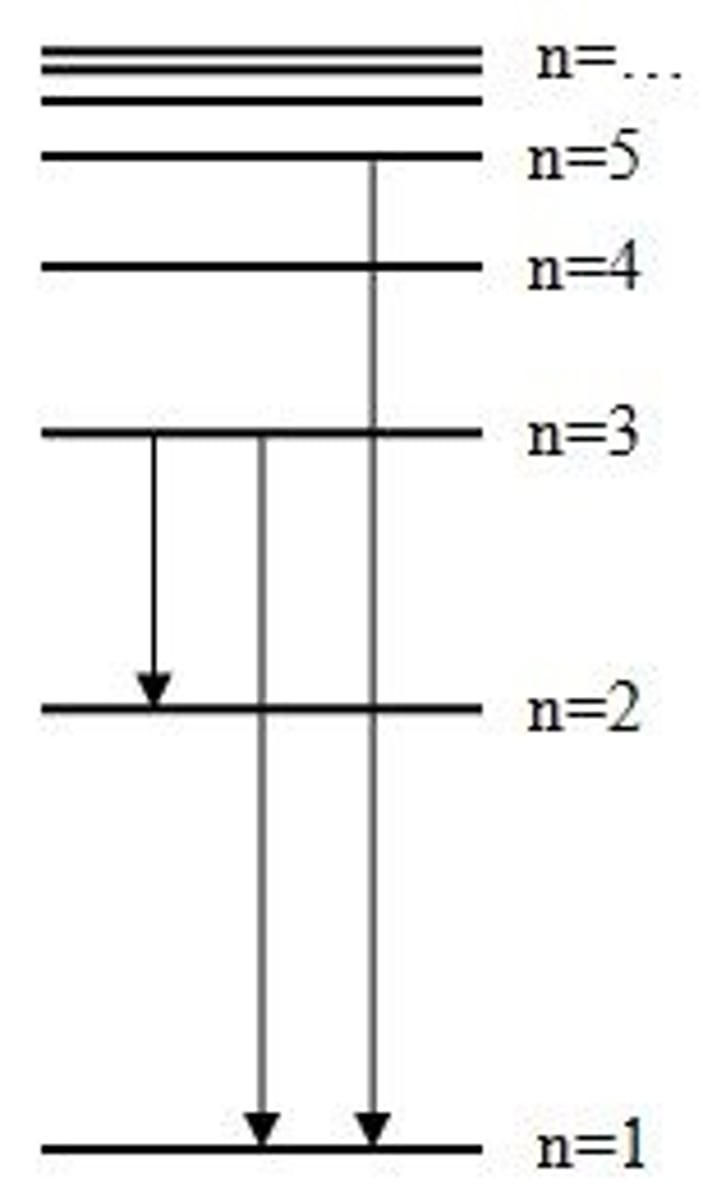
Hydrogen energy levels
- electrons dropping to n=1 -> ultraviolet light
- electrons dropping to n=2 -> visible light
- electrons dropping to n=3 -> infrared light
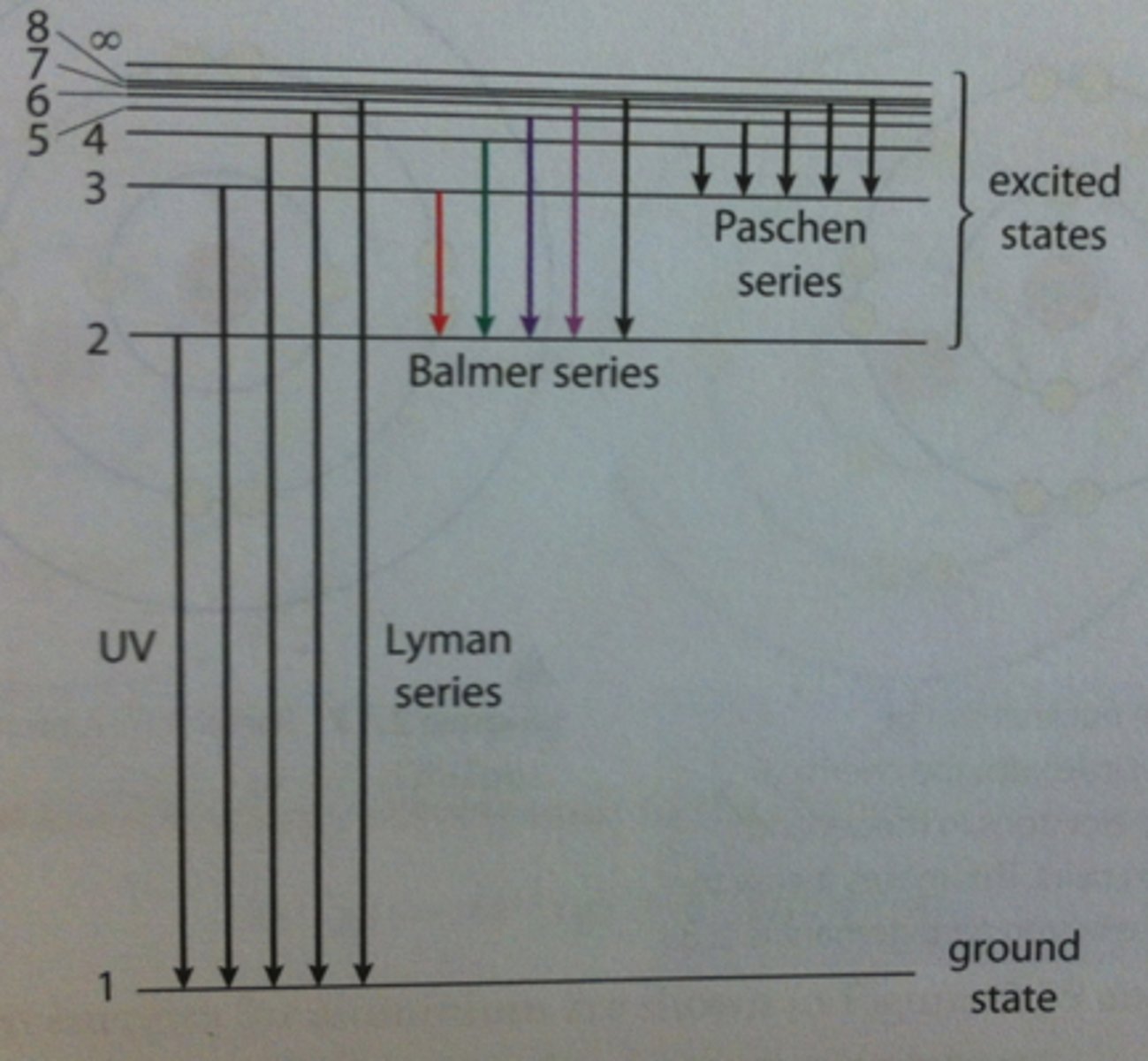
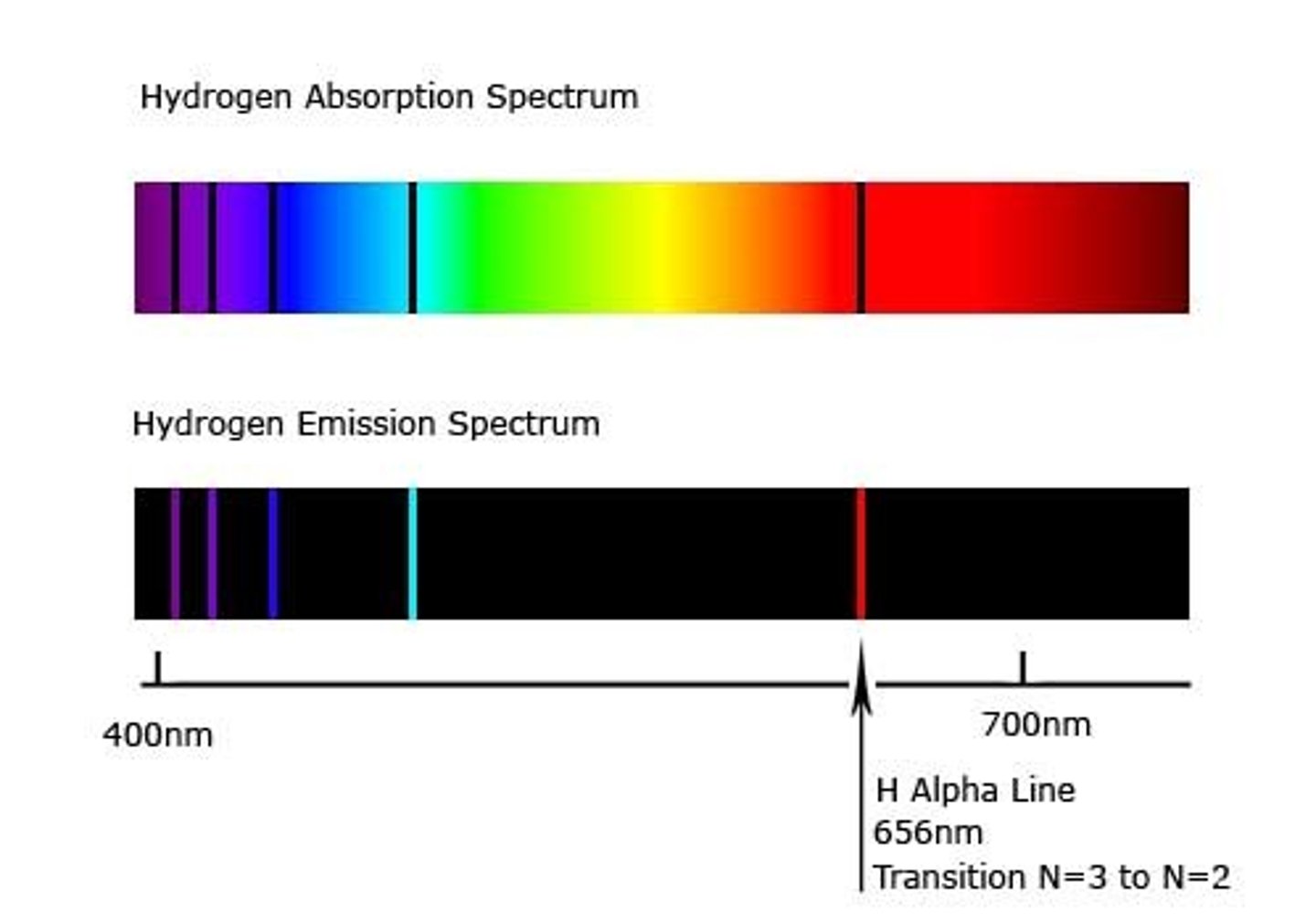
Hydrogen electron dropping to n=2 release ____ colours.
FOUR
violet, blue, blue-green, and red (right to left)
Convergence
Energy levels get tighter together the further away from the nucleus they are. The farthest energy levels have the most energy.

Emission spectrums can be used to figure out the type of ____ in a substance
ELEMENTS
this is because each element has its own unique emission spectrum due to the differing amounts of energy released as its electrons return to ground state.
Energy levels closer to the nucleus hold ____ electrons
FEWER
the maximum # of electrons in any energy level (n) is 2n^2
Why hydrogen?
Easiest element to study electron transmission patterns because each atom only has one electron
Atomic models
Representations of different theories showing what an atom may look like. Has been updated based on new information many times.
Hard to develop because atoms are so small.
Problems of the Bohr model
1. model couldn't predict the emission spectra of elements with more than one electron
2. it assumed the electron was a subatomic particle in a fixed orbit around the nucleus
3. model couldn't account for the effect of electric and magnetic fields on the spectral lines of atoms and ions
4. it couldn't explain molecular bonding and geometry
5. Contradicted Heisenberg's uncertainty principle
Heisenberg uncertainty principle
it is impossible to know exactly both the velocity and the position of a particle at the same time
Schrödinger wave functions
Describes the electrons by their probability density, using the Heisenberg uncertainty principle. Gives the probability that an electron will be found at a specific space from a certain distance from the nucleus.
Atomic orbital
a region of space in which there is a high probability (90%) of finding an electron.
Atomic orbitals are given a number to identify its energy level and a level to designate its shape
Ex. n=2, level=p -> 2p
Sublevels
Refers to electron orbitals designated s, p, d or f.
First four atomic orbitals
s, p, d, f
subsequent orbitals are theoretical, and are labeled alphabetically (g, h, i, k, ...)
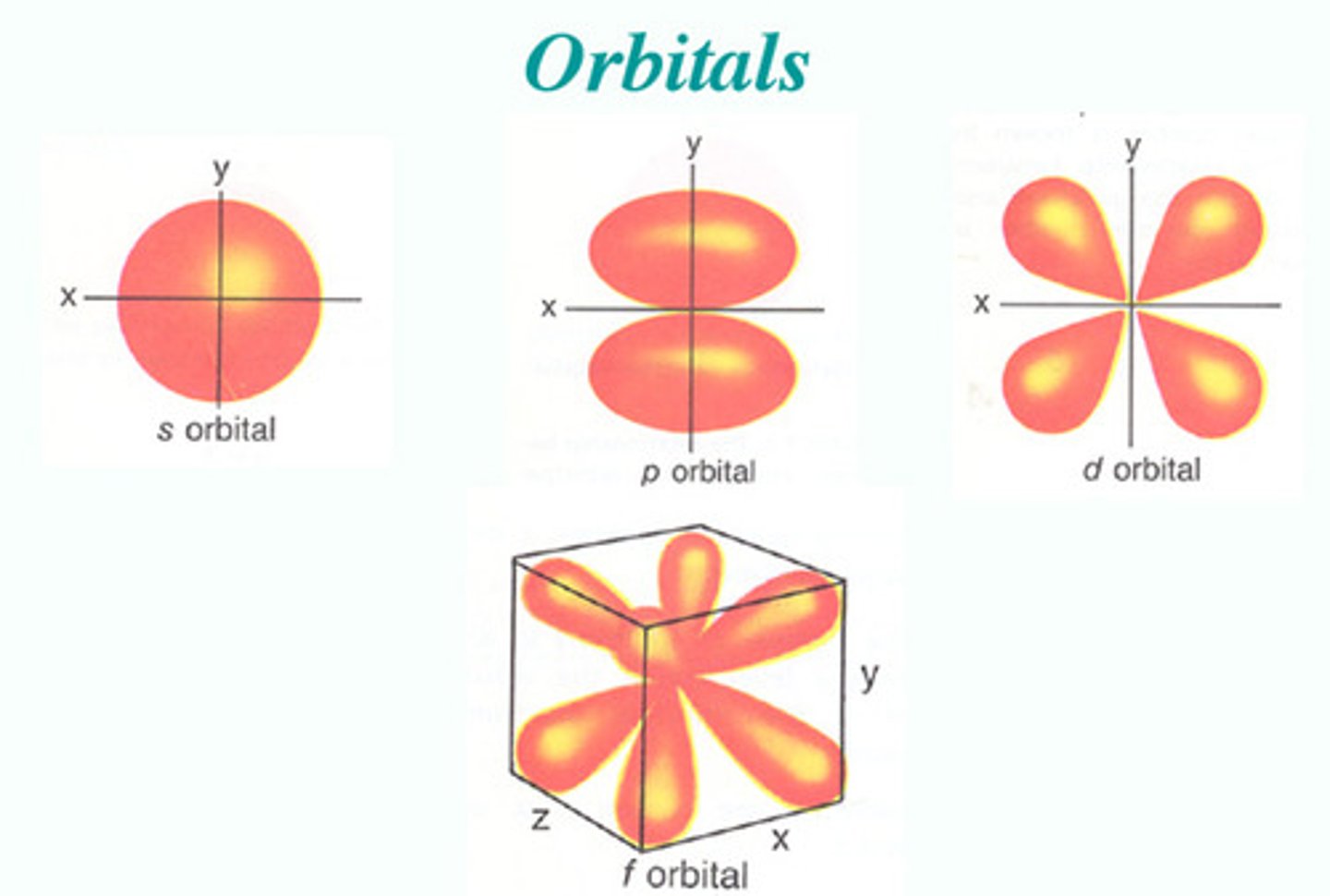
Each atomic orbital can hold up to____.
2 ELECTRONS
principal quantum number
symbolized by n, indicates the main energy levels surrounding a nucleus. The energy levels are split into sublevels containing atomic orbitals.
quantum mechanical model
the modern description, primarily mathematical, of the behavior of electrons in atoms. Based off of the probability of finding an electron at a specific space from a certain distance from the nucleus.
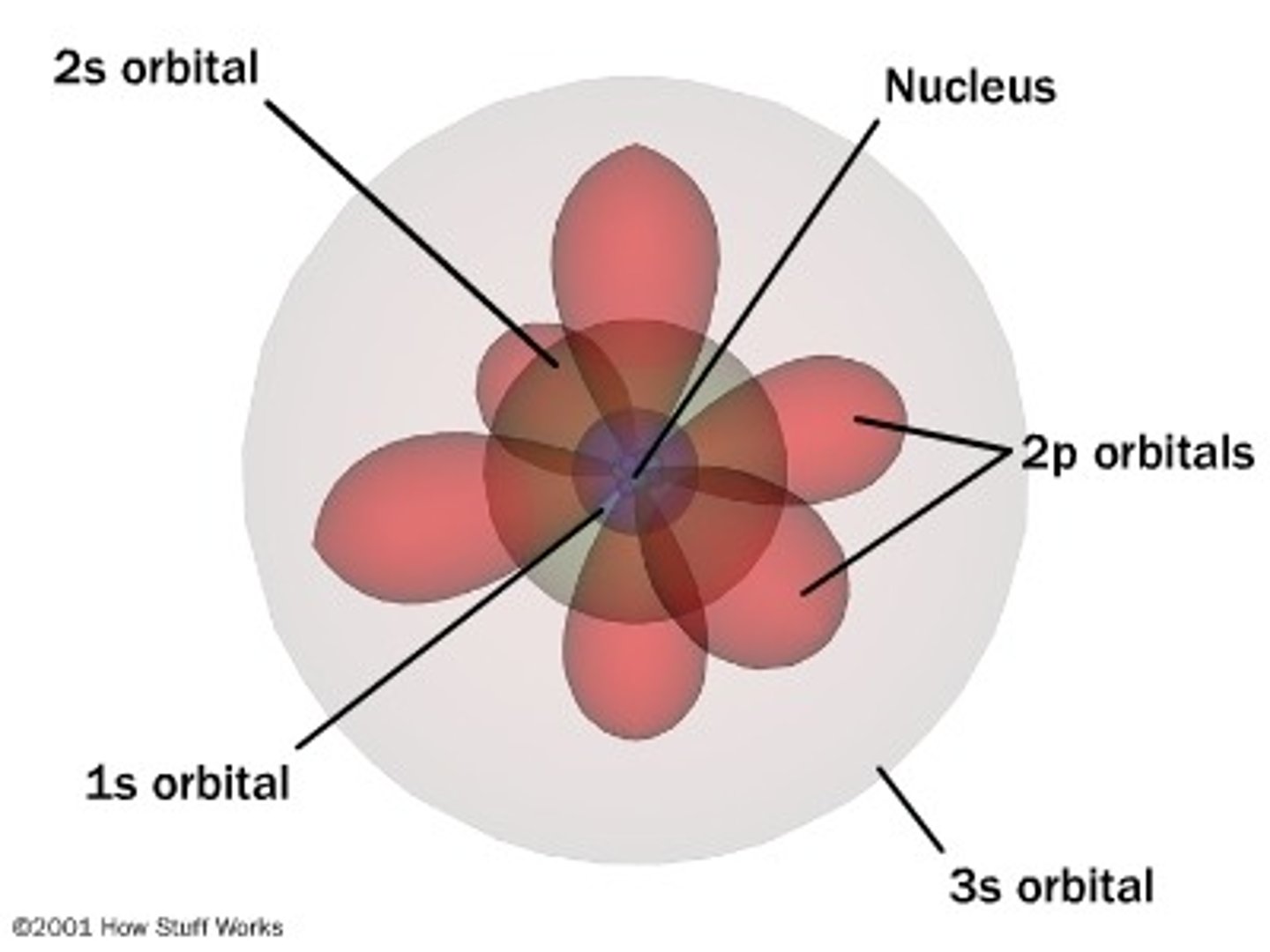
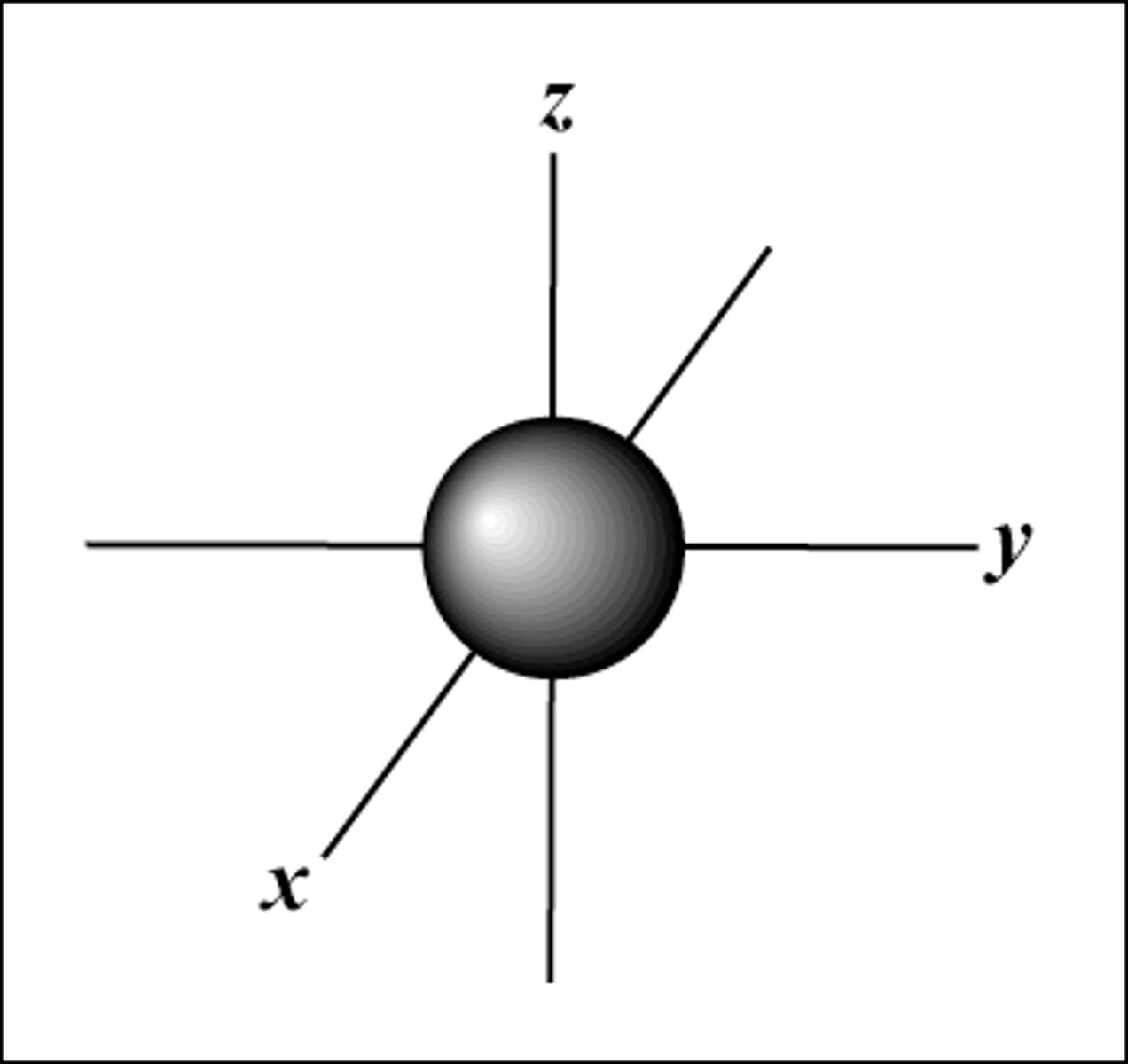
s-orbitals
- spherical in shape
- one s-orbital for each energy level
- increases in size with increase in energy level
- each s orbital contains 2 electrons
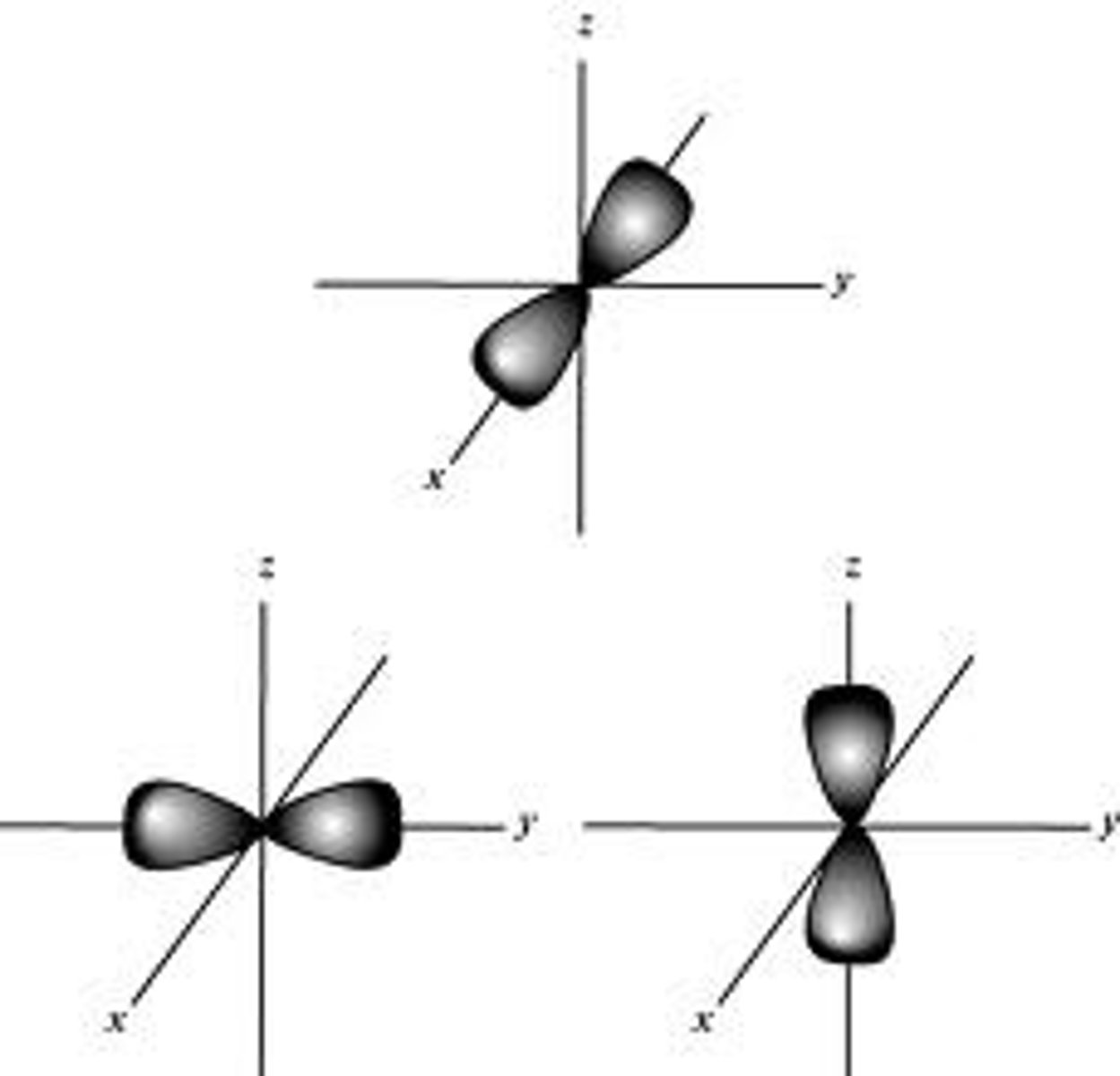
p-orbitals
- dumbbell shaped
- three p-orbitals along the x, y, and z axis
- no p-orbital in n=1
- each p-orbital holds 2 electrons, for a total of 6 electrons (3 axis * 2 electrons = 6 electrons)
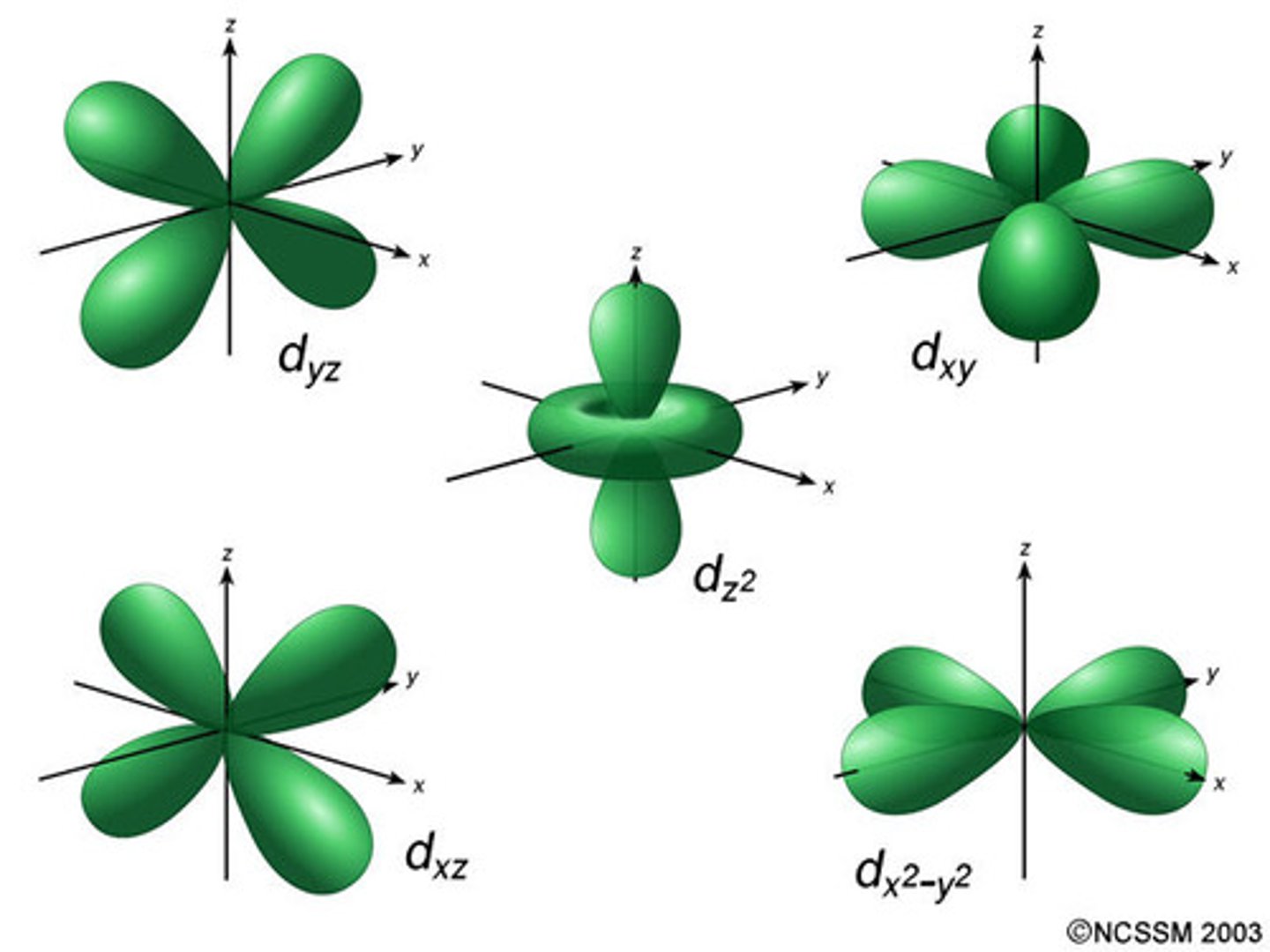
d-orbitals
- first d-orbital is in n=4
- d-sublevel made up of 5 atomic orbitals
- each holds 2 electrons for a total of 10 electrons
(5*2=10)
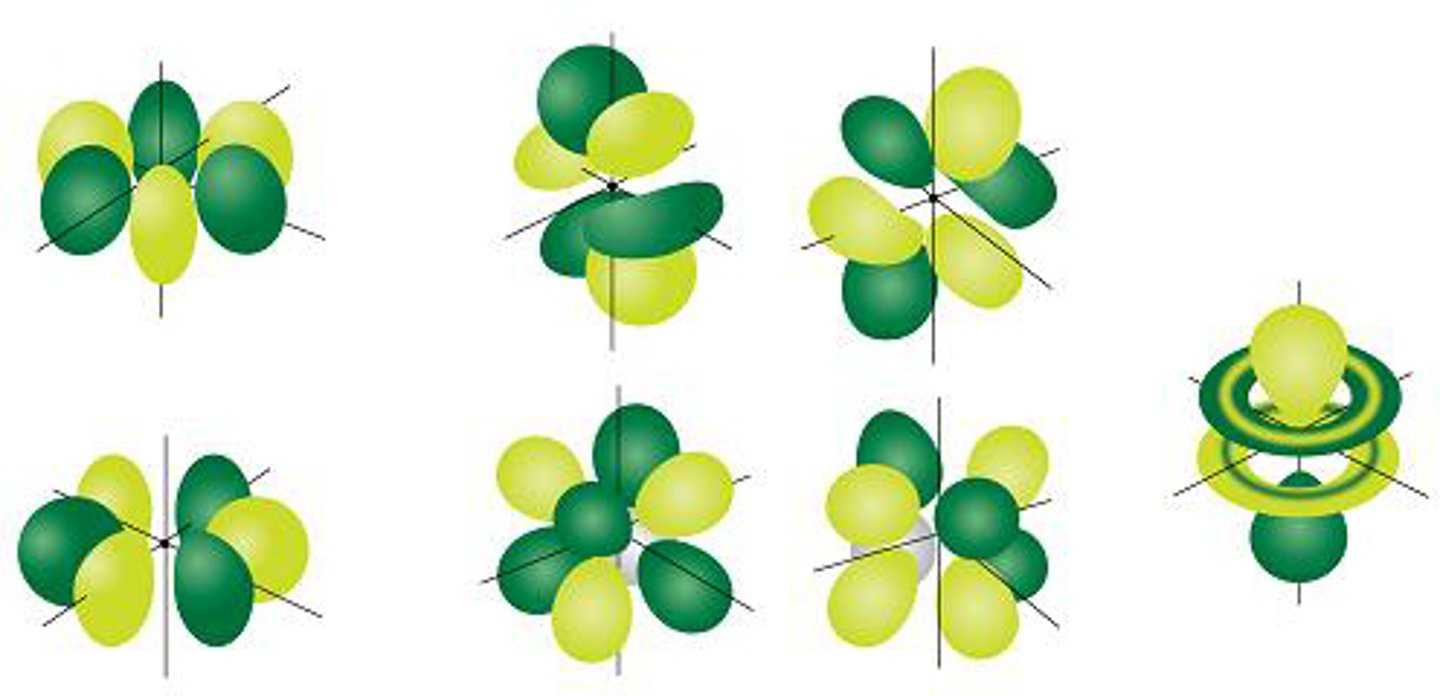
f-orbitals
- First f orbital in n=6, but assigned as 4f orbital (n-2)
- f-sublevel made up of 7 f orbitals
- each holds 2 electrons for a total of 14 electrons (2*7=14)
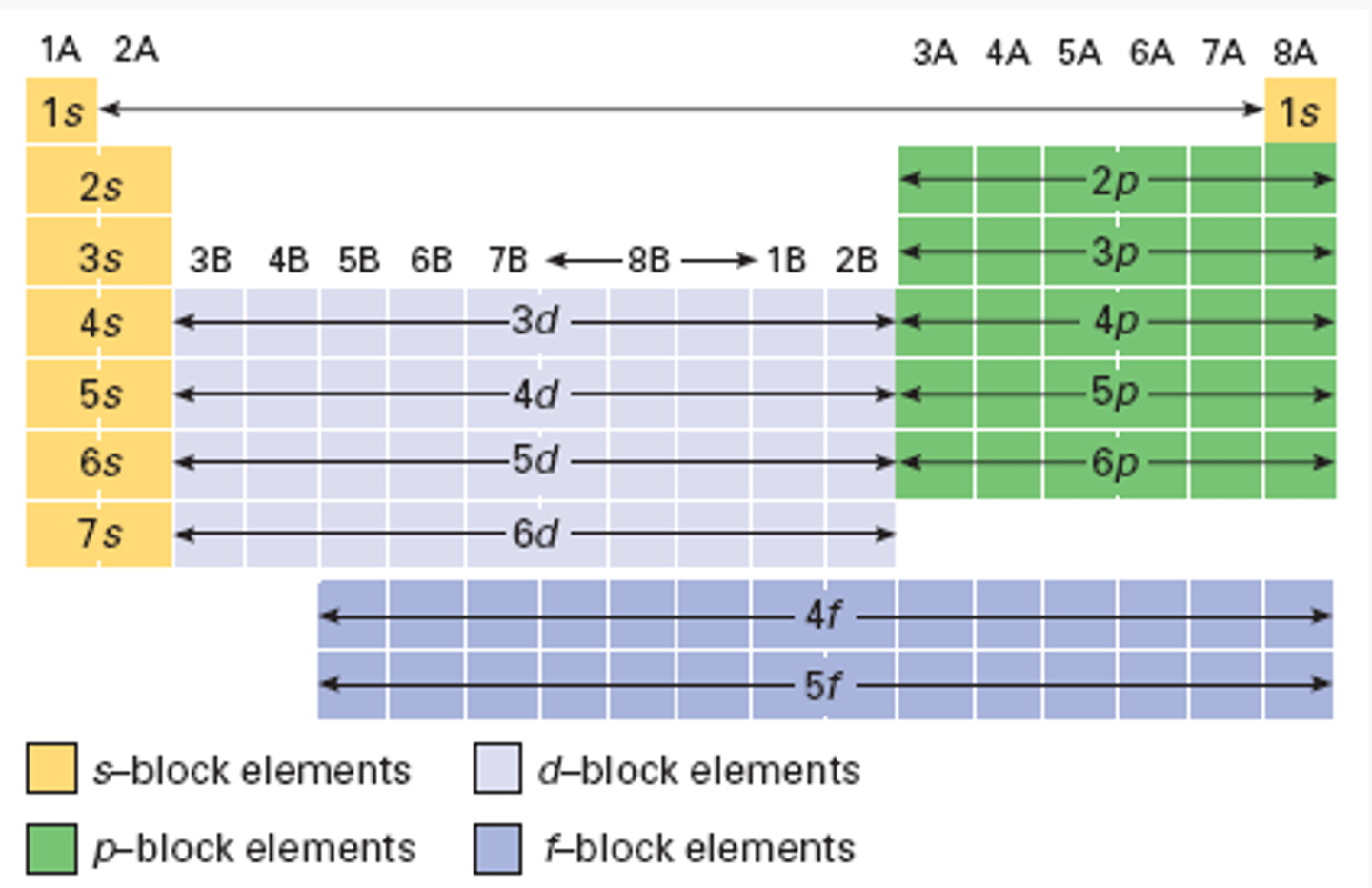
atomic orbital groups on periodic table
Things to take note of:
- He is part of the s-orbitals
- The d-orbitals are n-1 (Ex. n=4 -> 3d)
- The f-orbitals are n-2 (Ex. n=6 -> 4f)
The number of atomic orbitals at nth level is ____
n^2
(n = energy level)
In the nth level there are ____ sub-levels
n (s, p ,d, f...)
Ex. n=1 -> s-sublevel
n=3 -> s, p, and d sublevels
(n = energy level)
The number of electrons at nth level is ____.
2n^2
(n = energy level)
Orbital diagram
a diagram that shows the distribution of electrons in the orbitals of the energy levels.
Each atomic orbital in a sublevel has a separate box that can hold up to 2 electrons.
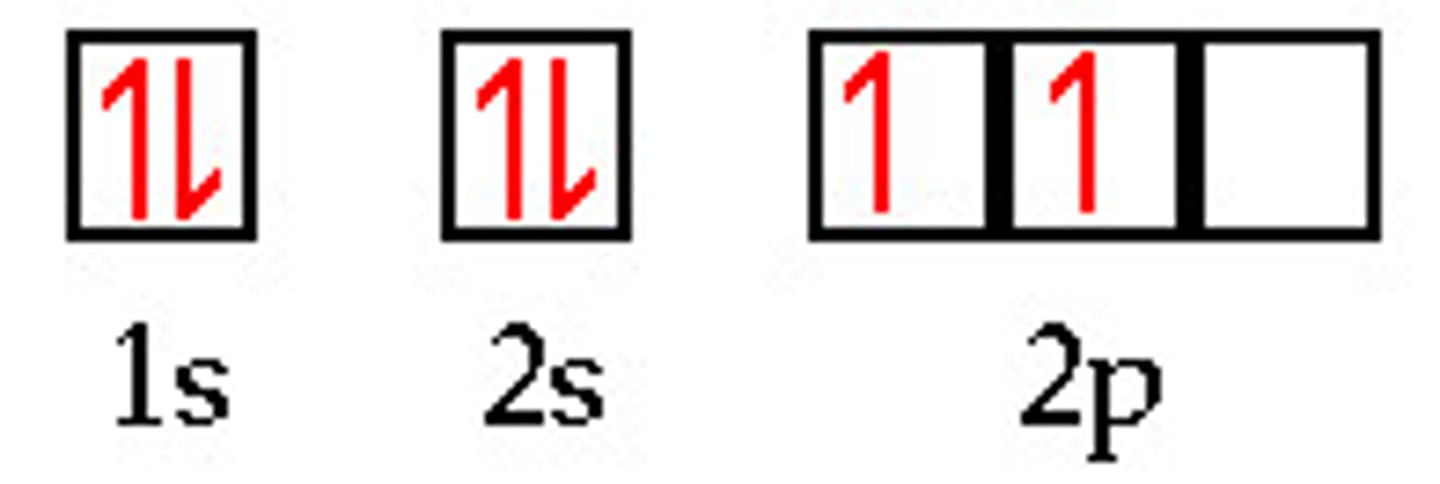
Electron configuration
the arrangement of electrons in orbitals (in an atom)
Pauli Exclusion Principle
Only two electrons can occupy the same atomic orbital and those electrons must have opposite spins.
Why?
- electrons have a negative charge, and like charges repel each other
- with opposite spins, the electrons are like magnets facing in opposite directions -- they don't repel
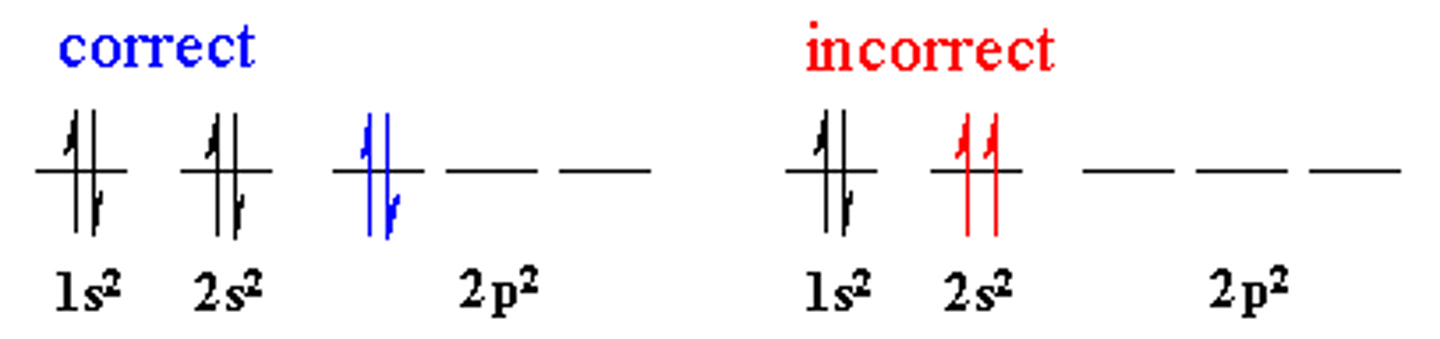
degenerate orbitals
orbitals that have the same energy
Ex. the three orbitals in the p sublevel
Hund's rule
When electrons occupy orbitals of the same energy, one electron enters each orbital until all orbitals contain one electron with spins parallel.
Only after this condition is fulfilled can you add electrons of opposite spin.
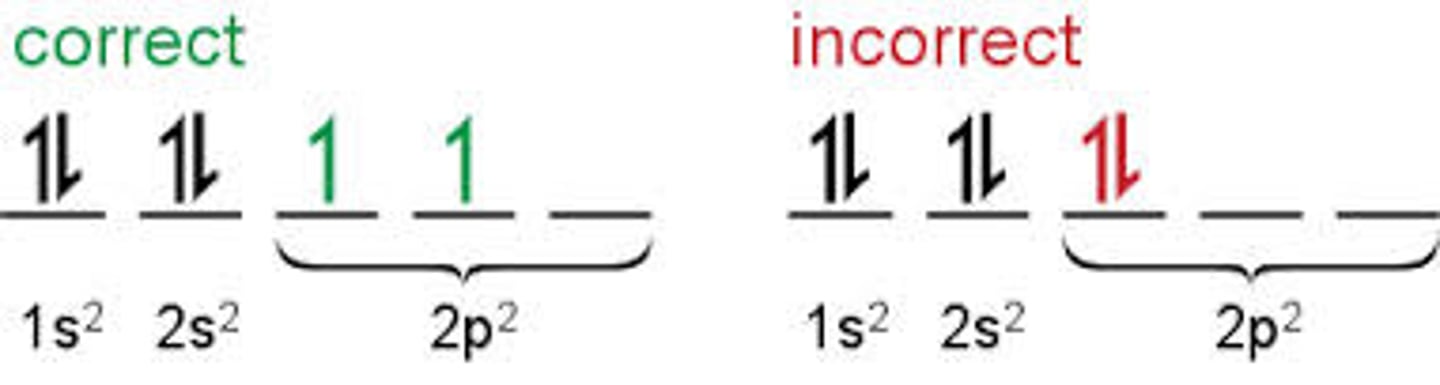
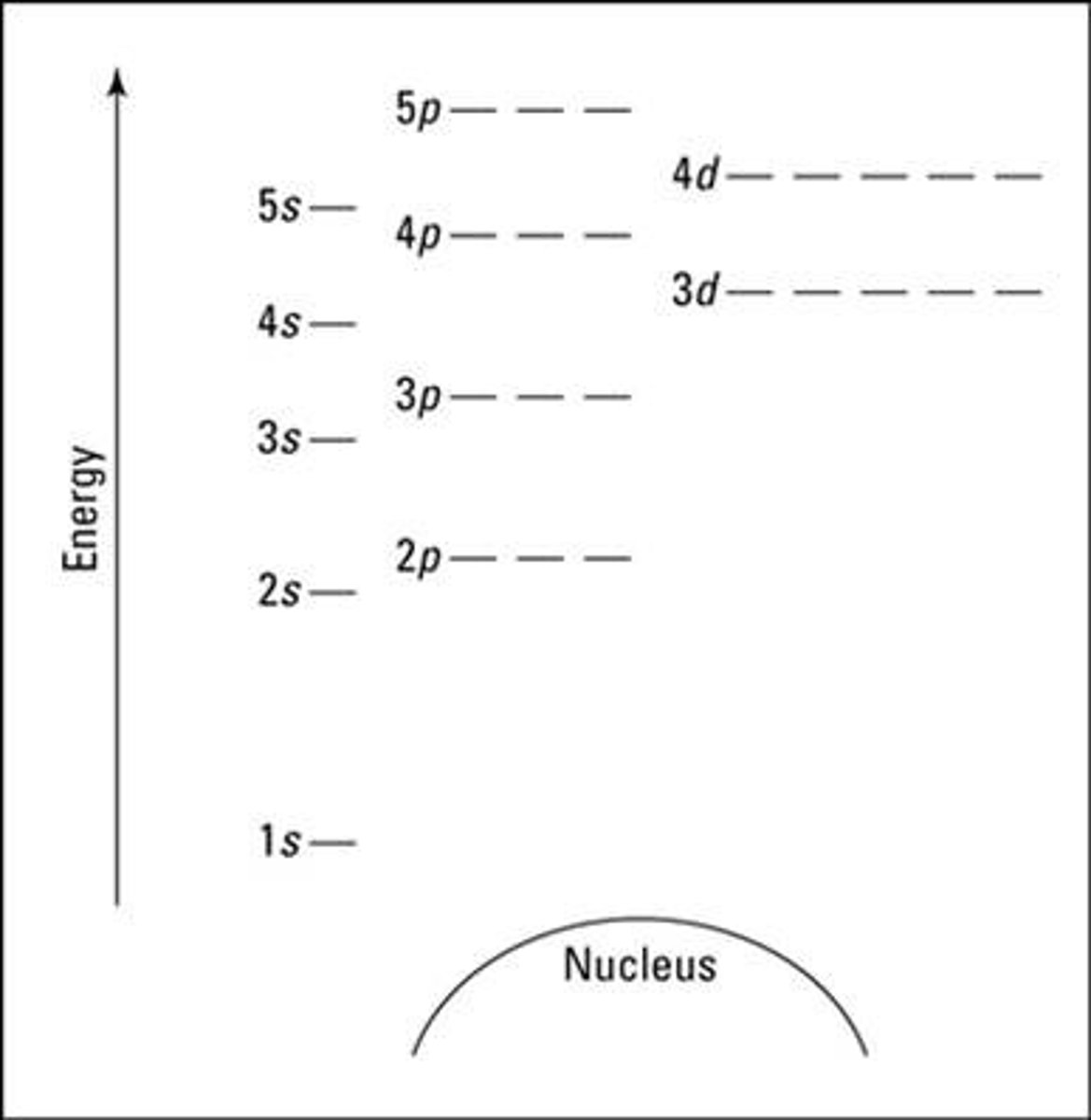
Aufbau Principle
An electron occupies the lowest-energy orbital first (closest to the nucleus). Orbitals within a sublevel have equal energy.

Exceptions for aufbau principle
Copper (Cu) and Chromium (Cr) + all the elements in their column
Why?
- copper promotes one of its 4s electrons to 3d in order to have a full 3d orbital. This makes the electron more stable
Expected configuration: [Ar]4s^2 3d^9
Observed configuration: [Ar]4s^1 3d^10
- chromium promotes one of its 4s electron to 3d in order to have 6 half filled orbitals with spins parallel. This makes the electron more stable
Expected configuration: [Ar]3d^4 4s^2
Observed configuration: [Ar]3d^5 4s^1
full electron configuration
type of electron configuration that contains energy level, orbital type, and superscript in one long line
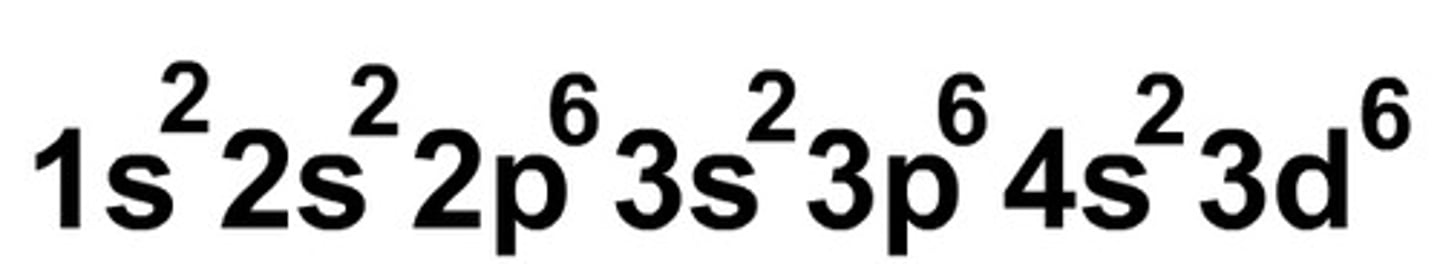
Condensed electron configuration
[previous noble gas] + valence electrons
![<p>[previous noble gas] + valence electrons</p>](https://knowt-user-attachments.s3.amazonaws.com/22124e2e-c5db-4e86-b703-ca6051d71c8d.jpg)
Inner (core) electrons
electrons that fill all the energy levels of an atom except the valence level; electrons also present in atoms of the previous noble gas
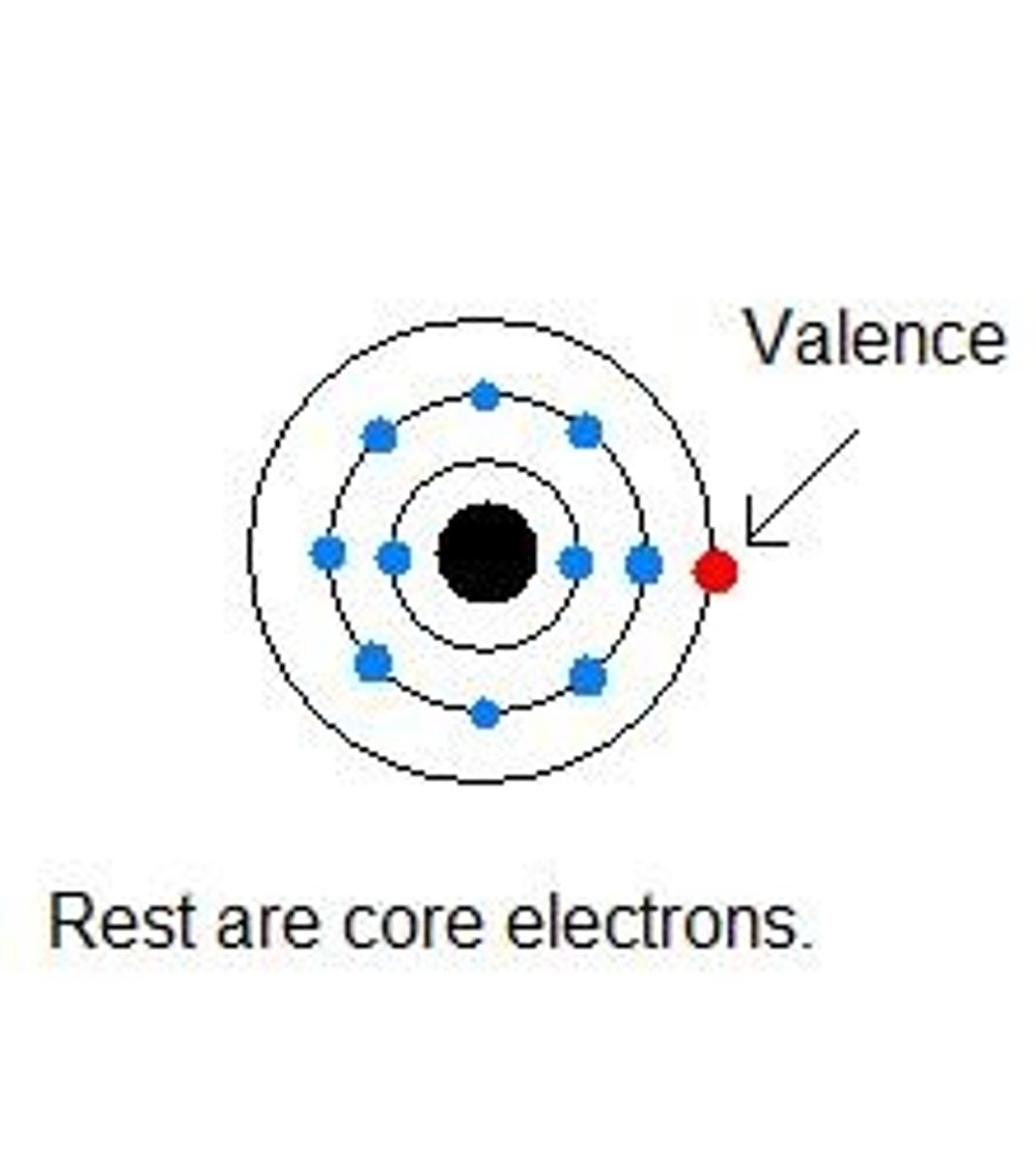
Outer electrons
electrons in the highest energy level (highest n)
Valence electrons
Electrons (in the outer shell) involved in forming compounds
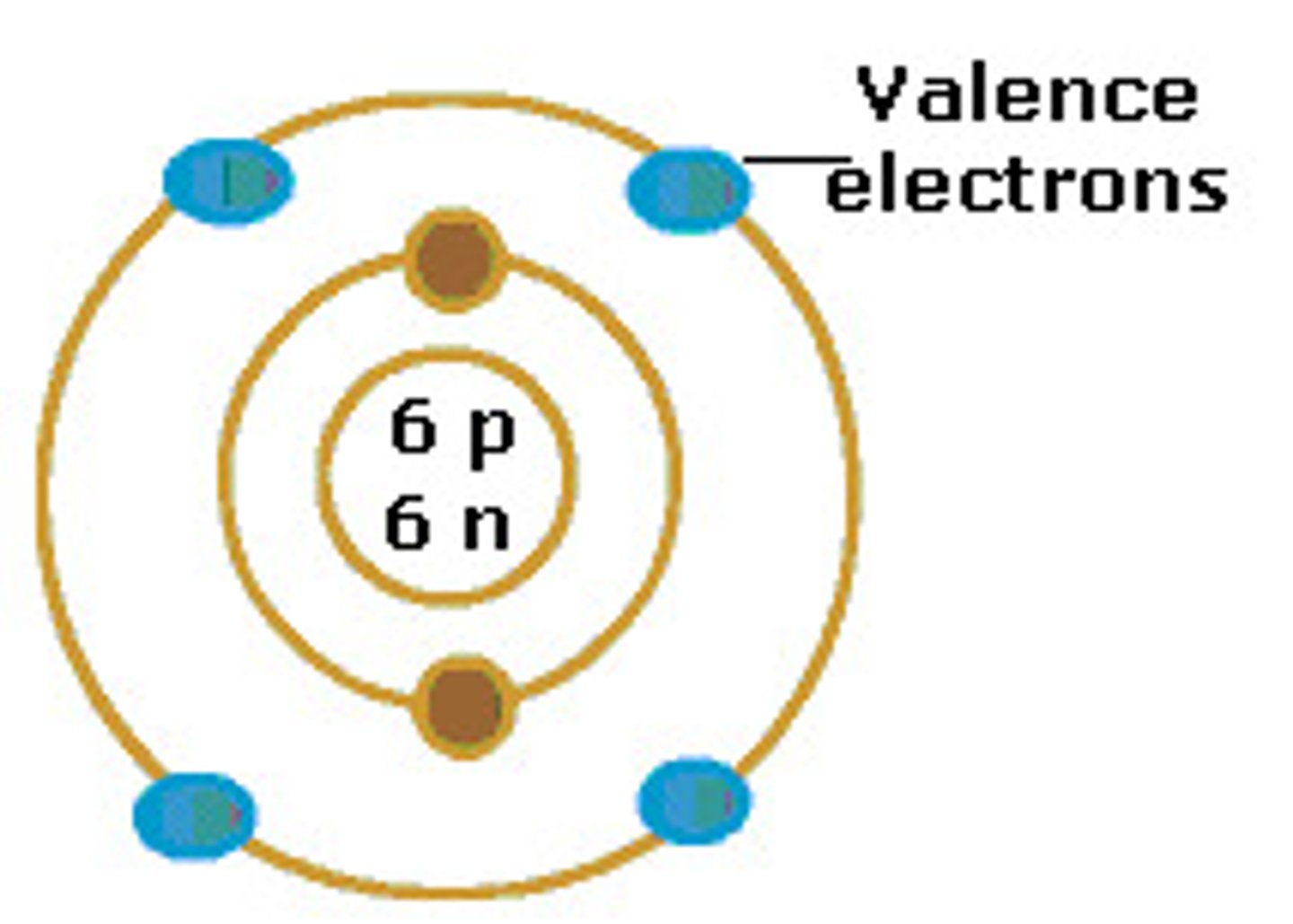
In the representative elements (s and p blocks), outer electrons ARE the ____.
VALENCE ELECTRONS
In the transition elements (d and f block) the outer electrons AND the ____ are considered valence.
D-ELECTRONS
Positive ion configuration
outermost level lose electrons depending on the charge.
Ex. Al -> 1s^2 2s^2 2p^6 3s^2 3p^1
Al+ -> 1s^2 2s^2 2p^6 3s^2
Negative ion configuration
The electron(s)(depending on the charge) is added to the next available orbital.
Ex. P >> 1s^2 2s^2 2p^6 3s^2 3p^3
P^3- >> 1s^2 2s^2 2p^6 3s^2 3p^6
Electron configurations can help chemists predict a lot of information about ____ and explain ____.
ATOM BEHAVIOR, BONDING