BME 33 MIDTERM 1
1/224
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
225 Terms
Light microscope
use visual light to illuminate specimens: was used to identify cells for the first time
- can be used to visualize cells in isolation or in a tissue
electron microscope
use beams of electrons as the source of illumination, visualizing fine details of cells down to single molecules
- higher resolution organelles, plasma, and internal membranes
what is histology?
tissue processing
1. tissue biopsy
2. fixation - dehydration - embedding
3. section
H & E Stain
H and E staining helps identify different types of cells and tissues and provides important information about the pattern, shape, and structure of cells in a tissue sample.
Hematoxylin (purple): stains cell nucleus
Eosin (pink): rest of cell and connective tissue
What is the central dogma?
genetic information flows only in one direction, from DNA, to RNA, to protein, or RNA directly to protein.
Describe prokaryotic cell
- bacteria and archaea
- unicellular
- lacking nucleus and organelles (no defined internal membranes)
- 0.1 - 10um
Describe eukaryotic cell
- animal and plant cells
- multicellular
- nucleus
- internal membranes, distinct organelles
- 10-100um
Describe nucleus
- control center of the cell
- manages all of the cell's activities
- largest organelle
- contains DNA
- surrounded by nuclear envelope
DNA is located in the ___ and protein is made in the ___
nucleus, cytoplasm
Single membrane organelles
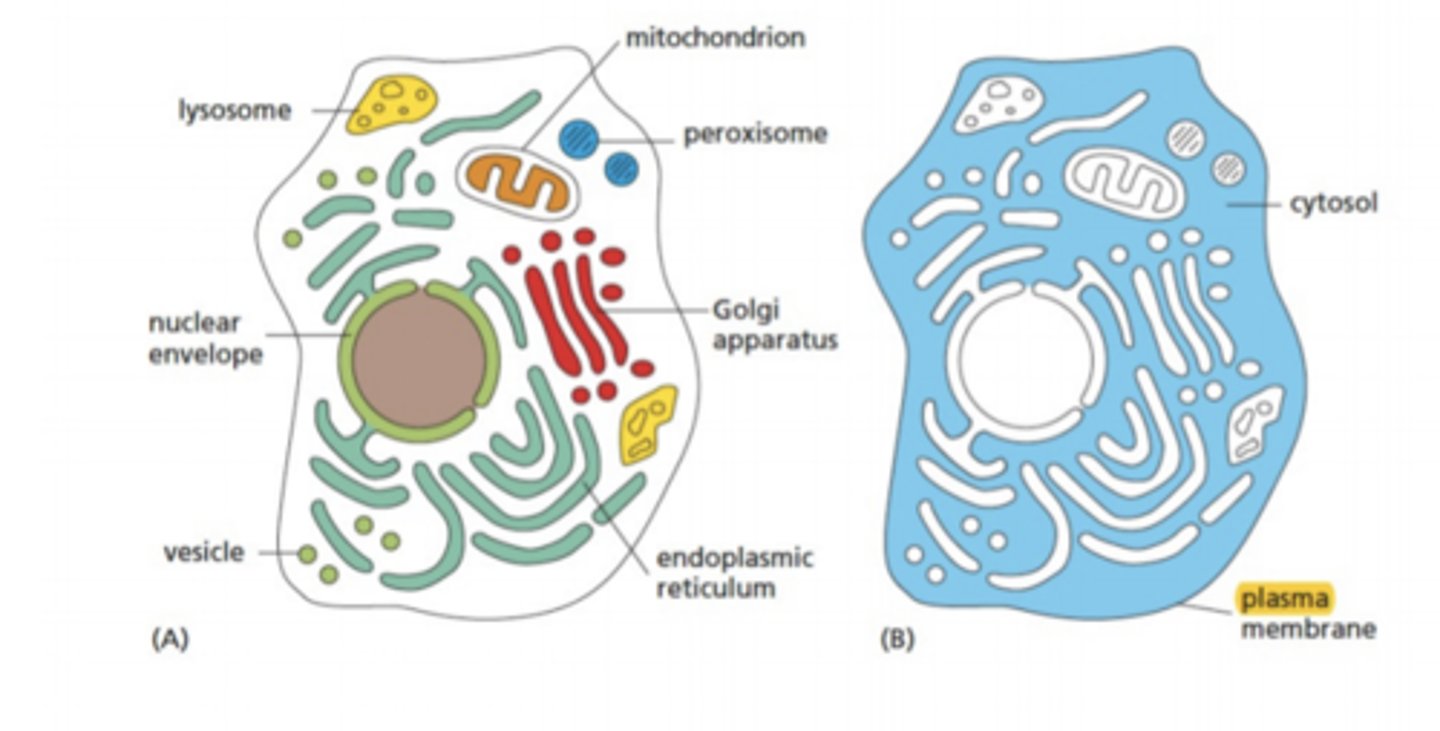
Cytoplasm
ALL cell contents within the cell membrane, not including nucleus
Cytosol
the aqueous portion contained within the cell, surrounding organelles
Manufacturing: protein synthesis and transport
ER
golgi
vesicles
Digestion
lysosomes
peroxisomes
Energy
mitochondria/chloroplasts
Endoplasmic Reticulum
- network of channels leading from nuclear membrane into cytoplasm
- Rough ER: sited of protein synthesis by ribosomes and folding
- smooth ER: has no ribosomes, involved in lipid and hormone synthesis
Golgi
- assembled proteins are packaged in vesicles
- vesicles and functional proteins may be directed to another location I the cell or released extracellularly
Lysosomes
- membrane sac of hydrolytic enzymes
- lysosomal enzymes break down proteins, fats polysaccharides and nucleus acids
- used to digest nutrients coming into the cell and also to recycle cell's own organelles, macromolecules
Peroxisomes
- small membrane enclosed vesicles similar to lysosomes but the enzymes require oxygen to function
- play a role in digestion fatty acids, alcohol and other proteins
- provide a safe environment for reactions in which hydrogen peroxide is used to inactivate toxic molecule s
mitochondria
- in all eukaryotic cells
- generate usable chemical energy from chemical bonds in food and stores in ATP by cellular respiration
- two separate membranes
- variable number per cell, relating to cell function
- contains own DNA and divides in two
chloroplasts
- in cells of plants and algae
- mitochondria oxidize these sugars in ATP production
- both organelles contain their own DNA -> divide separately
Cytoskeleton
frame that gives shape to a cell
- helps cell move in environment and controls intracellular movement
- made up of actin, microtubules, intermediate filaments
Actin
maintains overall cell shape, muscle contraction, cell division, organelle movement
microtubules
contribute to cell motility, chromosomes and organelle movement
intermediate filaments
maintenance of cell shape, anchor nucleus
ECM
3D network of extracllular macromolecules that provide strucutual and biochemical support to surrounding cells
What stain is used for ECM?
Masson Trichrome
What is Masson Trichrome?
blue: collagen fibers
brown: cell nuclei
red: cytoplasm
Catabolism
breakdown of food into smaller molecules to generate useful form of energy and small molecules to be used as building blocks
Anabolism
use of energy harnessed by catabolism to drive synthesis of molecules inside the cells
cells are predominantly made up of which chemical elements?
CHON (99%)
- another 20 elements are an essential part of the 1%
covalent bonds
incorporate the sharing of electrons; commonly generated through condensation reaction (release of water) in carbohydrate, ATP, and peptide formation
**Strong bonds
ionic bonds
require the transfer of electrons between 2 atoms and associated by electrostatic interactions
***strong binds, but dissociate easily
Hydrogen bonds
form when a positively charged H (polar covalent) comes near a negative charged atom- typically O or N
***weaker bonds and easily broken
Van der Waals
force that is driven by induced electrical interactions between two or more atoms that are very close to each other
*** weakest of intermolecular interactions
rank bonds in terms of strength (highest to lowest)
covalent
ionic
hydrogen
van der Waals
what is a condensation reaction?
When two molecules bond through the loss of a water molecule.
what is a hydrolysis reaction?
A covalent bond is broken by adding a molecule of water.
What are carbohydrates?
- all share the formula (CH2N)m
- individual subunits are monosaccharides linking to form polysaccharides through glycosidic linkages
- utilized as a source of energy and can be linked for long term storage as glycogen or starch
Glucose vs Fructose
Early research suggests that glucose and fructose follow different metabolic pathways
- glucose is processed into energy or stored as glycogen in the liver or muscles
- fructose is metabolized into fat
Sugars with formula C6H12O6
- may be found as different monosaccharides: glucose, galactose, or mannose
- by changing the spatial arrangement at the atoms, these 3 are isomers- atomically the same, but chemically different species
polysaccarides
repeating monosaccharides
- can offer structural support in plants (cellulose, pectate), animals (hyaluronic acid, chitin) and bacterial cells (murein)
what are the functions of fatty acids?
• Energy storage, as triacylglycerol can be tapped for 6x more
energy than glucose by weight (bonds are energy-rich)
• Membrane structure
• Protecting against desiccation
• Insulating against cold
• Absorbing shocks
• Regulating cell activities by hormone actions.
What is Niemann-Pick disease?
- involves the break down and use of fats and cholesterol in the body- body is unable to break down lipids in lysosomes
- harmful amounts of lipids accumulate in the spleen, liver, lungs, bone marrow, and brain
- clinical features: liver disease, breathing difficulty, seizures, lack of coordination, inability to move eyes vertically
- no current treatment options
- liver histology (H&E stain) shows contrast between healthy and neimann-pick cells
Describe fatty acid structure
- insoluble in water as largely non-polar, not charged/neutral; soluble in fat, organic solvents
- assembled, the molecule is amphipathic with a hydrophilic carboxylic acid at the head and a hydrophobic hydrocarbon tail
How are triacylglycerols formed?
by the attachment of 3 fatty acid chains to a glycerol molecule through ester linkages (through a process known as esterification)
where are fatty acids stored?
in the cytoplasm of cells as an energy reserve through an ester linkage to glycerol to form triacylglycerols
- as a food reserve, fatty acids as tricylglycerol can be tapped for 6x more energy than glucose by weight
What are saturated fats?
- pack together very well to potentially form plaques in blood (not healthy)
- accumulate cholesterol in blood stream
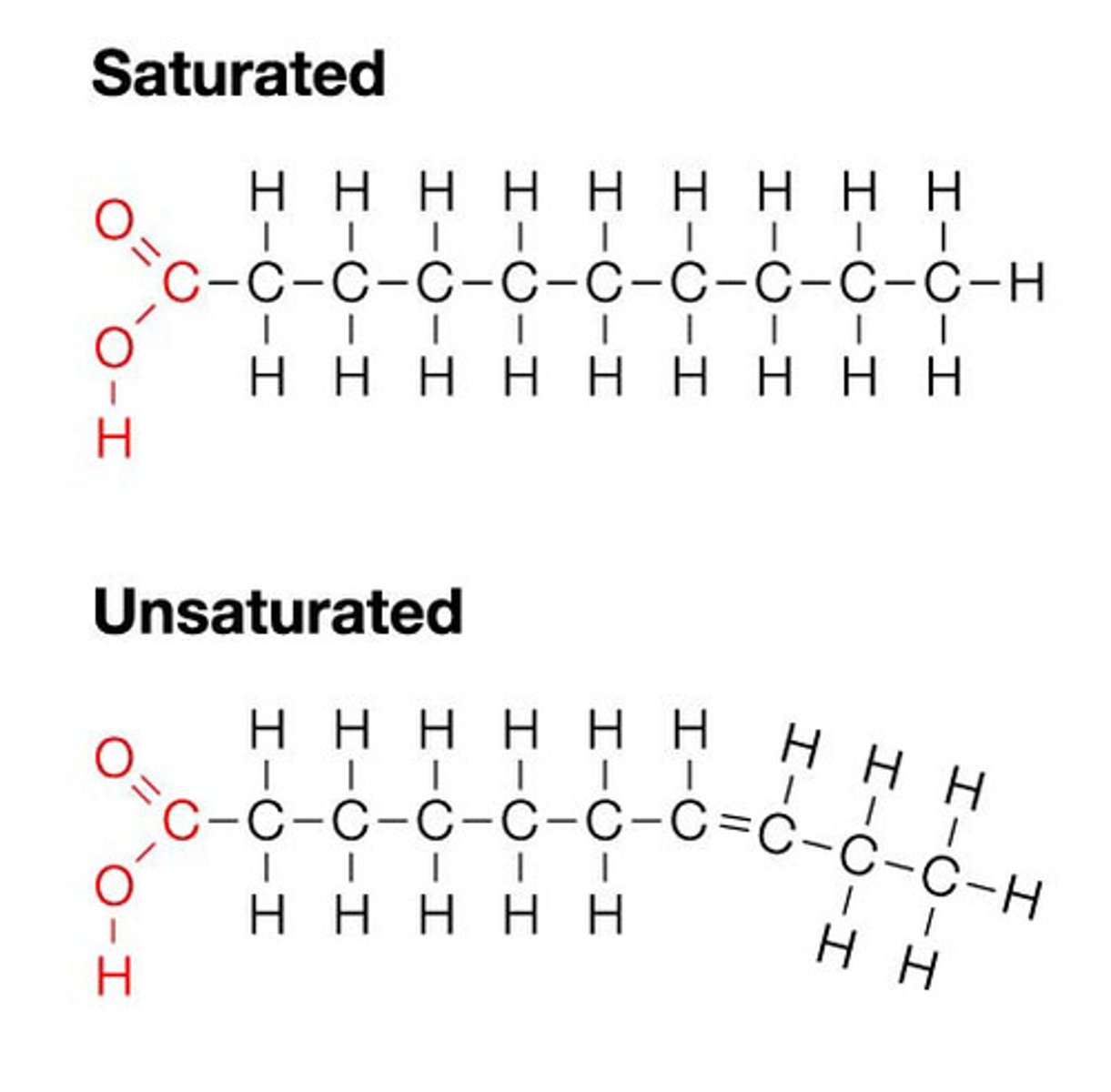
What are unsaturated fats?
- cis double bond cannot pack together as well due to stiffness with double bond (healthy)
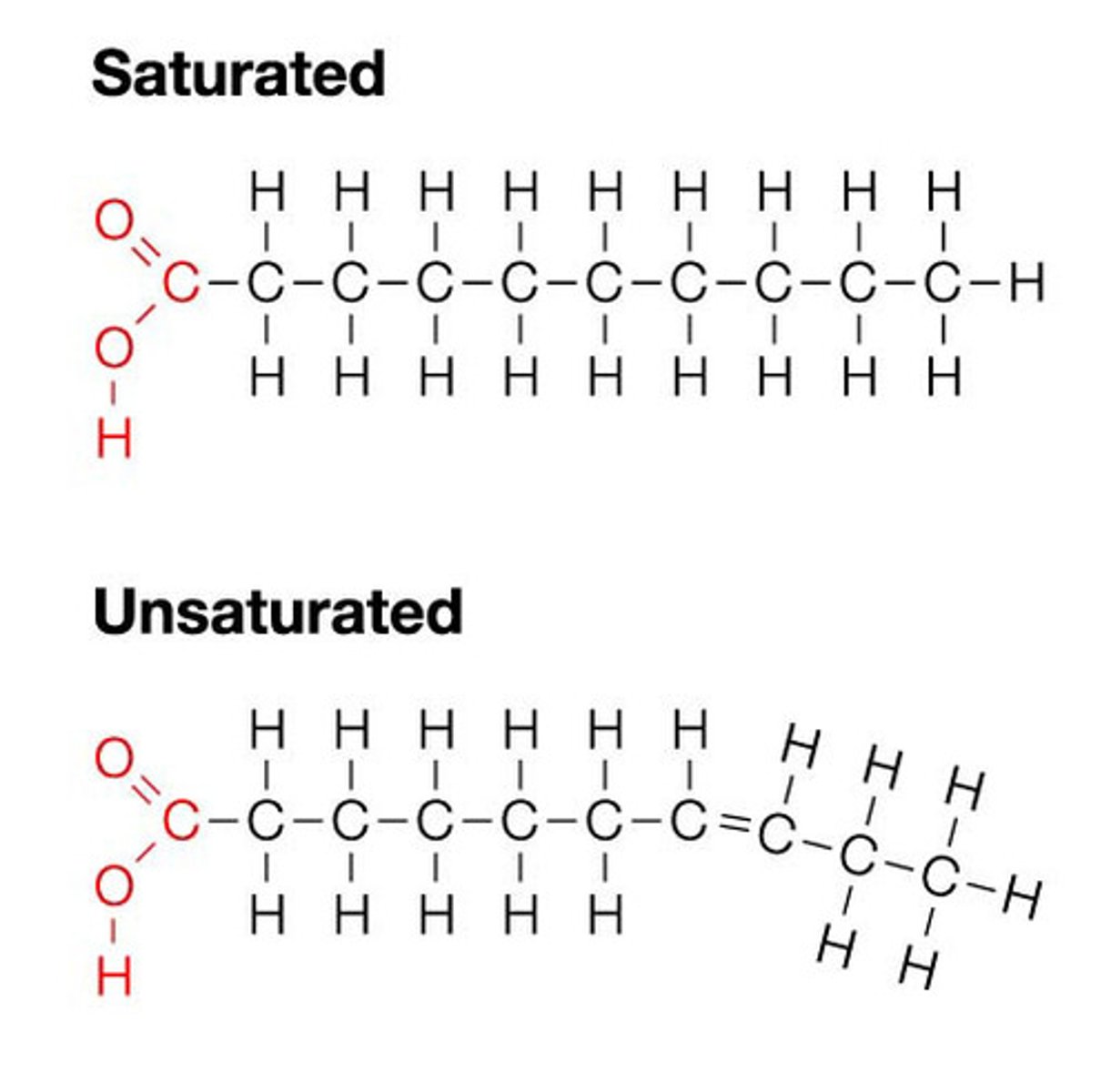
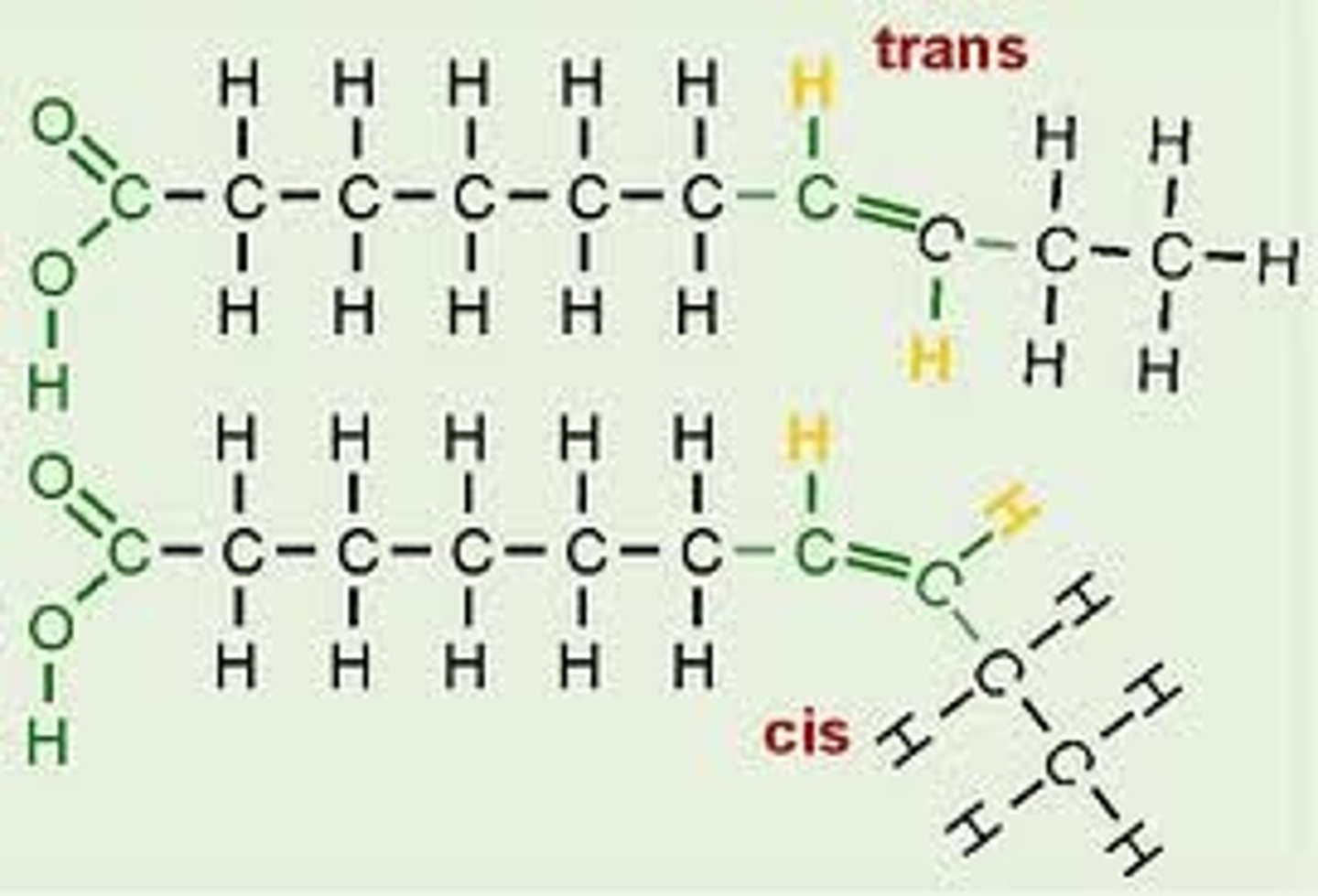
What are trans fats?
- have trans-bond; stack well, not well broken down as not natural
- 2% increase in trans-fats leads to a 23% increase risk of getting a heart attack
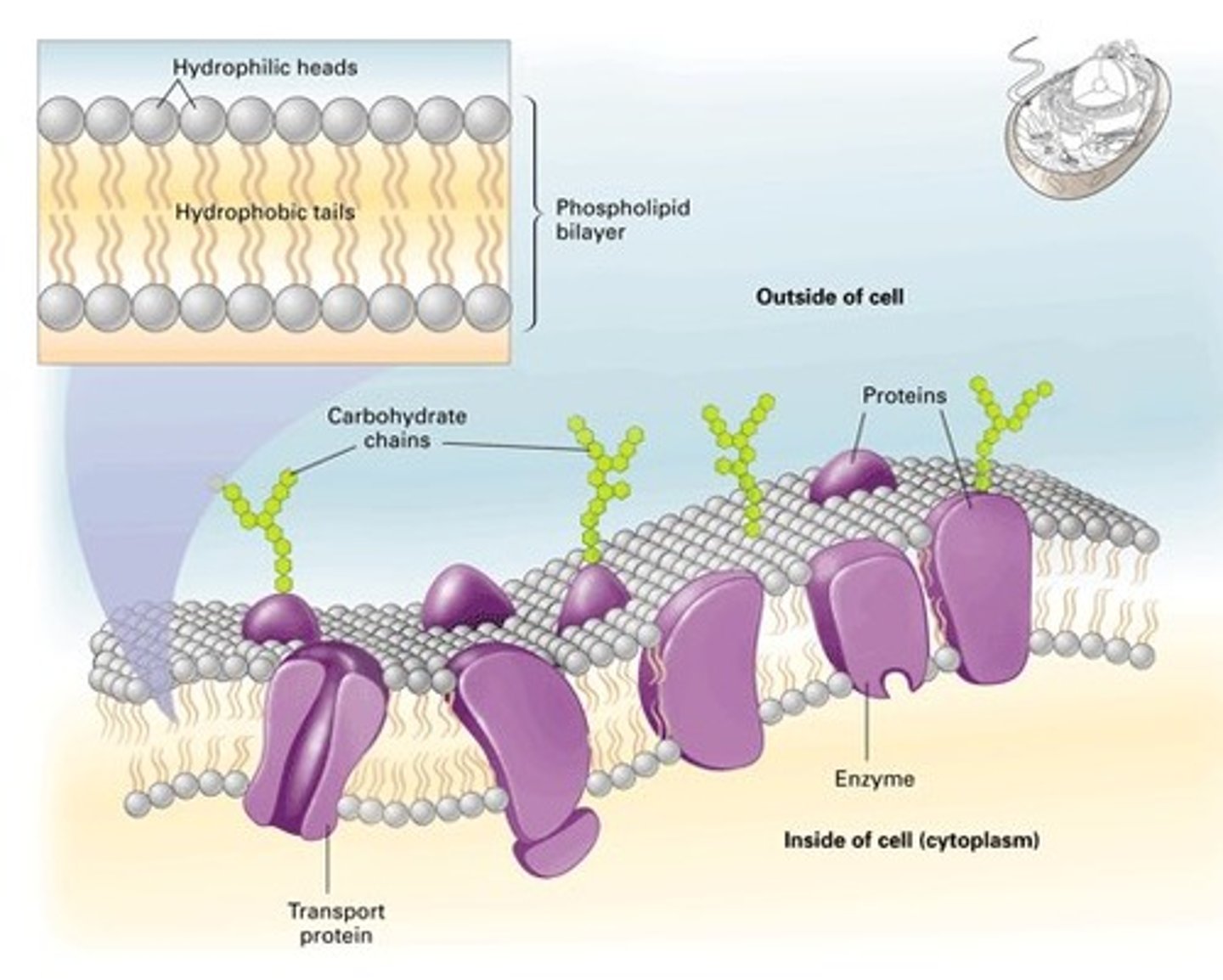
How do fatty acids contribute to membrane structure?
- phospholipids are constructed mainly from fatty acids and glycerol
- the glycerol is joined to two fatty acid chains, rather than three as in triacylglycerols
- the remaining -OH group on the glycerol is linked to a hydrophilic phosphate group, which in turn is attached to a small hydrophilic compound such as choline
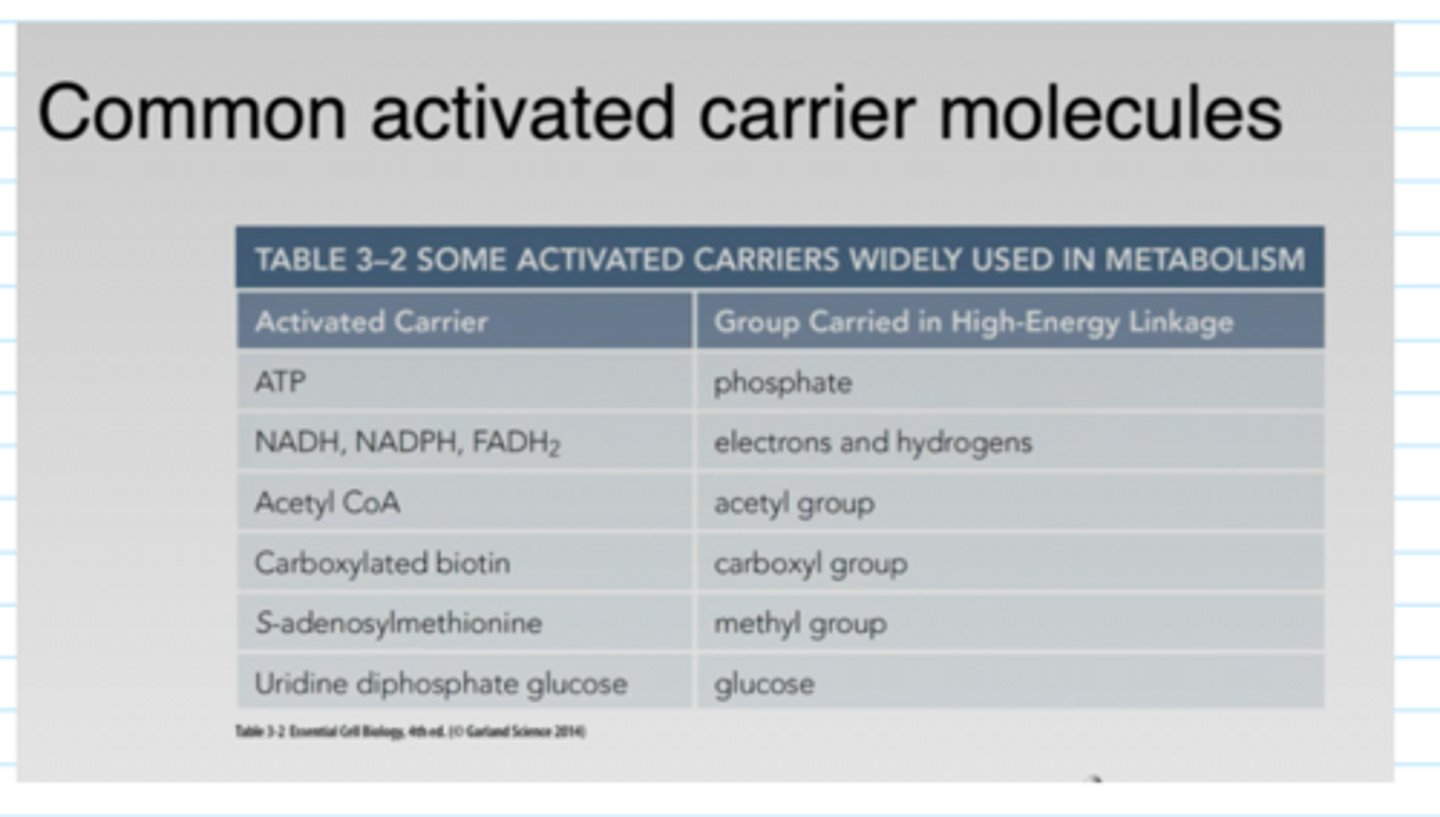
What are carrier molecules?
- energy released by the oxidation of food molecules must be store temporarily before it can be channeled into the construction go the many other molecules needed by the cell
- in most cases, the energy stored as chemical-bond energy in a small set of activated "carrier molecules" which contain one or more energy-rich covalent bonds
- activated carrier molecules perform their function as go-betweens that link the breakdown of food molecules and the release of energy to the energy-requiring biosynthesis of small and large organic molecules
What are some common carrier molecules?
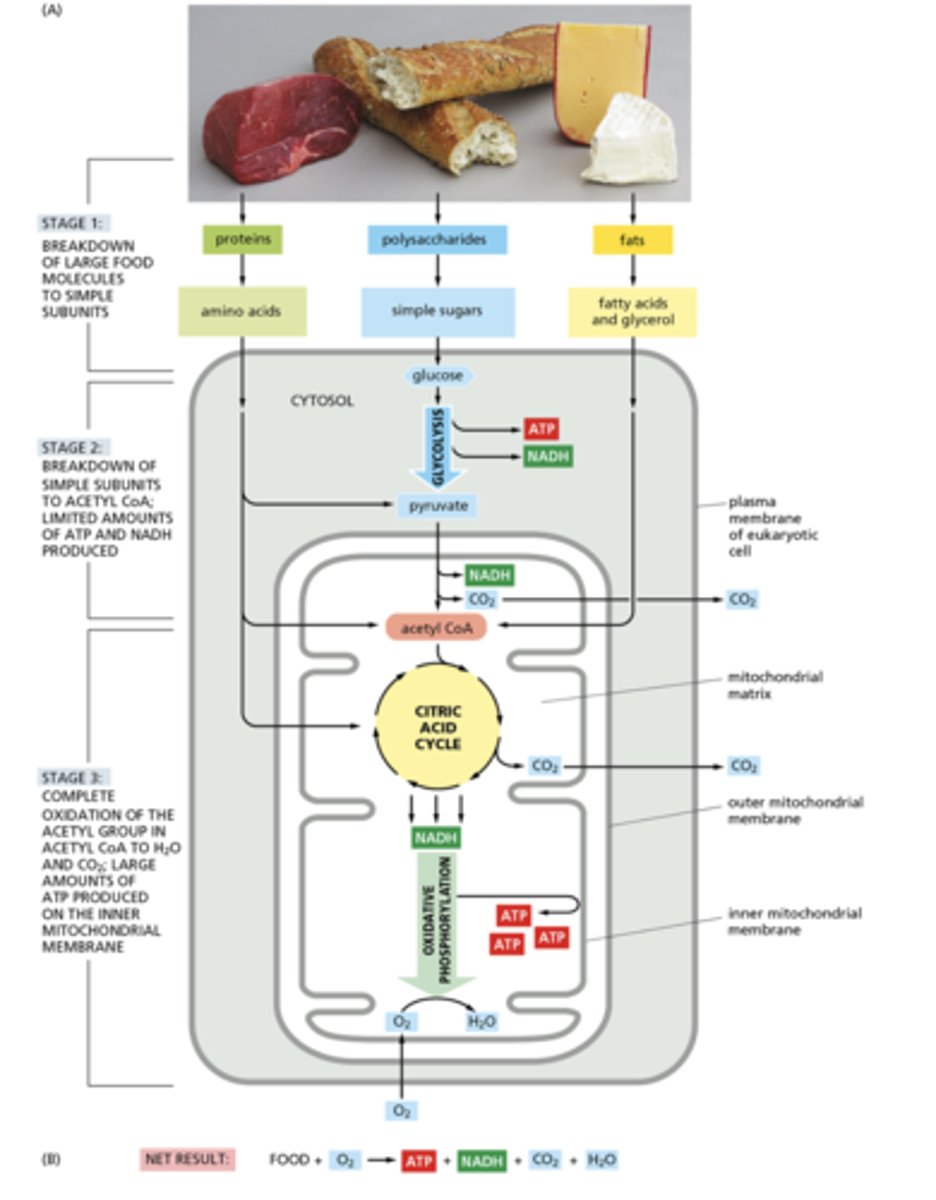
What are the three steps of catabolism?
1. Digestion to monomers- occurs in intestines outside cells or lysosomes
2. Breakdown of simple subunits to Acetyl CoA, limited amounts of ATP and NADH are produced
3. Complete oxidation of acetyl CoA to H2O and CO2 and production of large amounts of ATP, involves Citric Acid Cycle and ETC
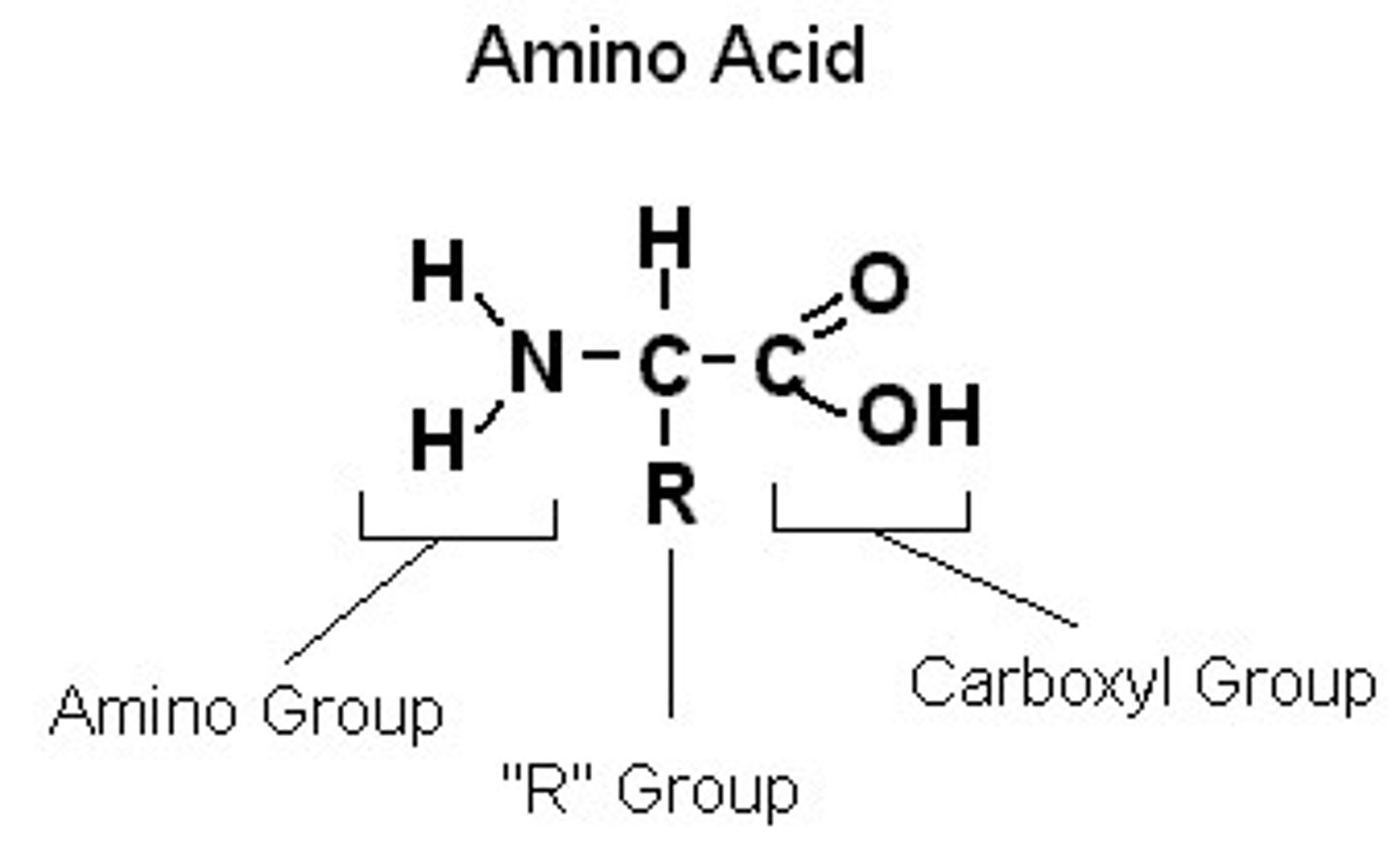
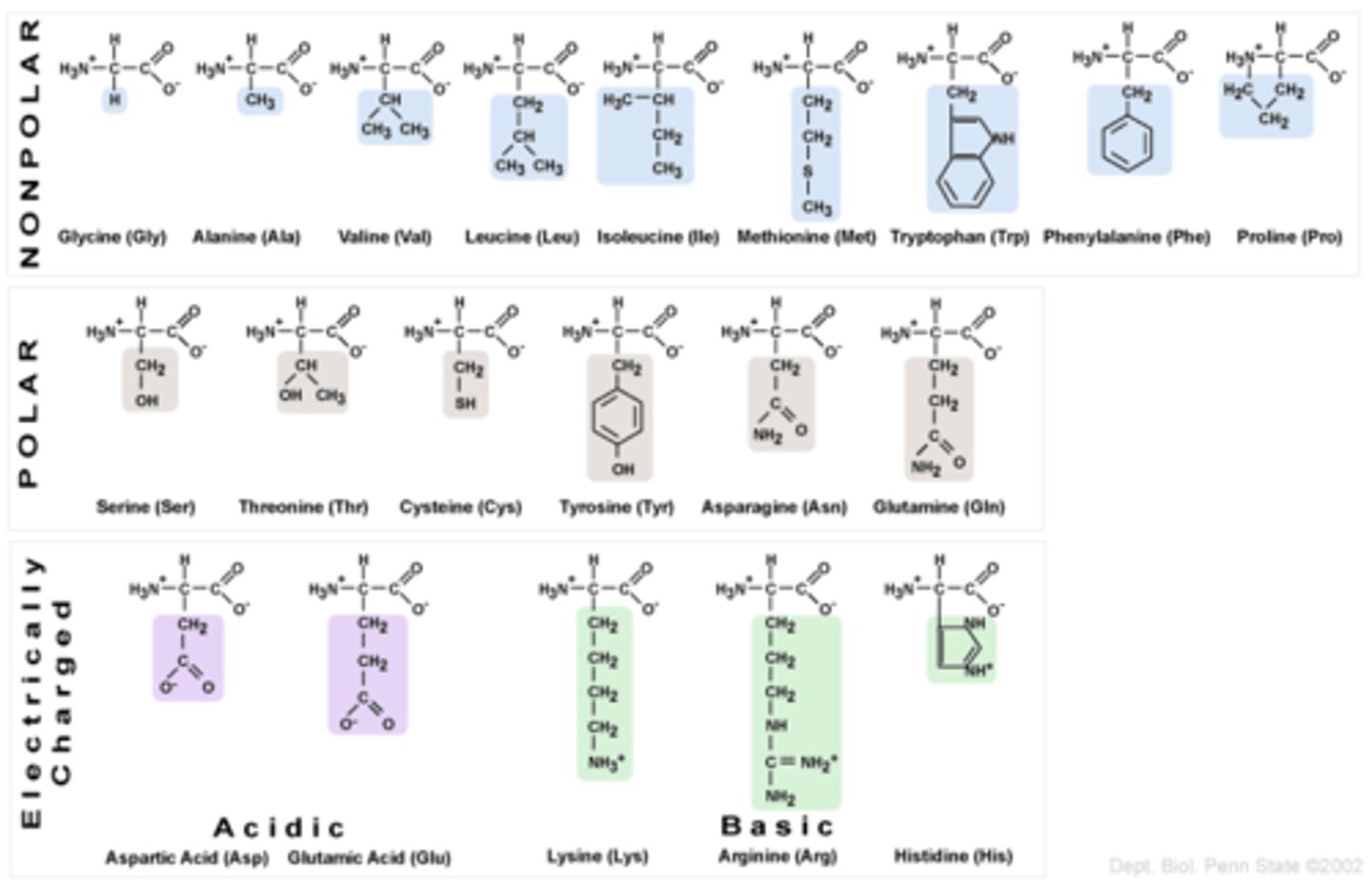
What are amino acids?
- subunit of peptides/proteins
- all share the same backbone of a carboxylic acid group and amino group linked to an alpha carbon
Describe Amino Acid Familes
- 20 amino acids utilized in the universal genetic code
- each represented by both 3- and 1- letter abbreviations
- additional, nonessential HAs have been identified in archaea and in derived culture conditions
What is phenylketonuria?
- syndrome involving impaired metabolism of the amino acid phenylalanine. PKU is caused by absent or virtually absent phenylalanine hydroxylase enzyme activity
What are peptide bonds?
-join individual AA's via a condensation reaction leading to peptides-polypeptides-proteins
Describe protein structure
- primary: orders of AAs
- secondary: the coiling of the primary sequence of AAs (includes beta-sheet and alpha-helix)
- tertiary: interaction of secondary coils to form 3D structures
- quaternary: complex of multiple proteins bound together
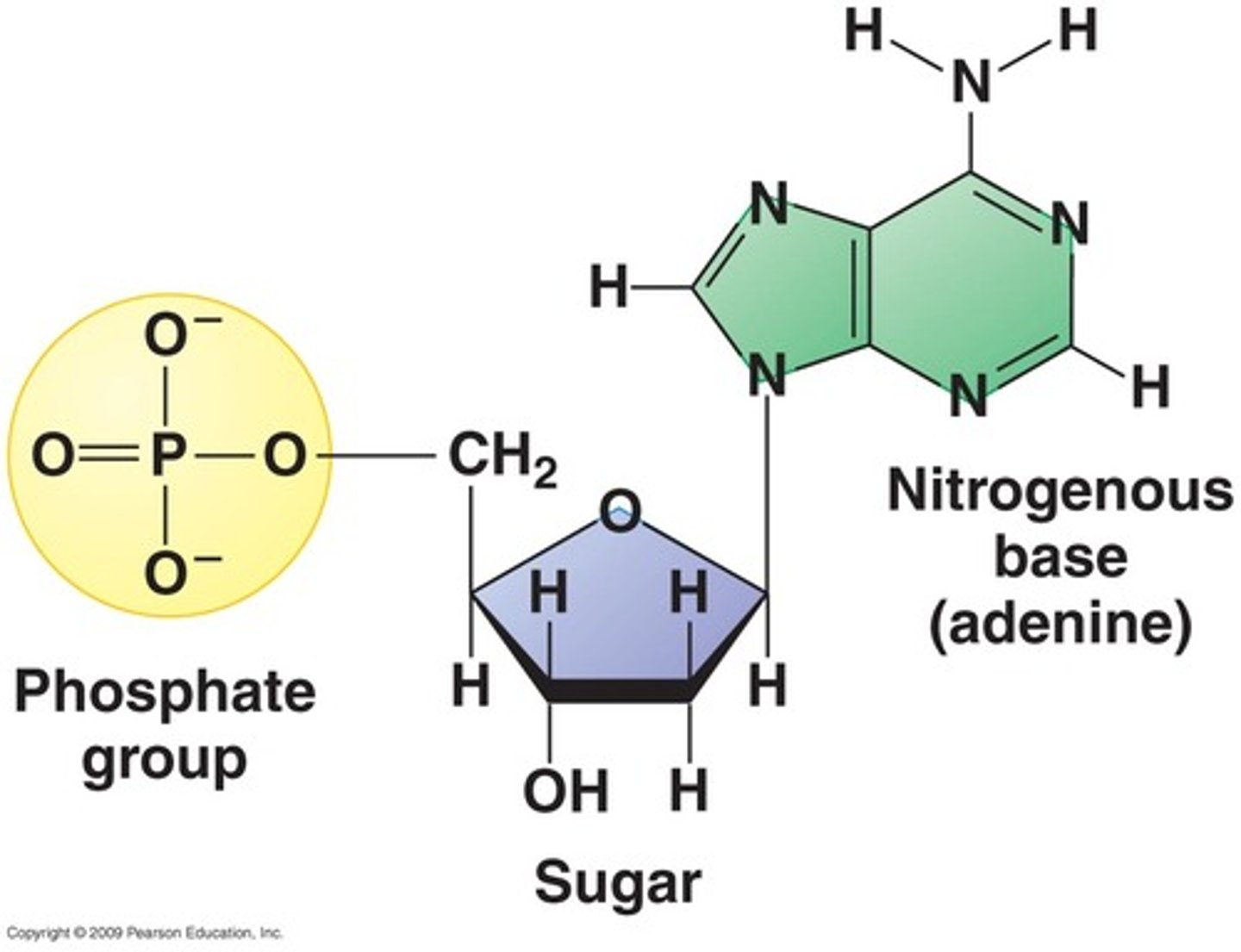
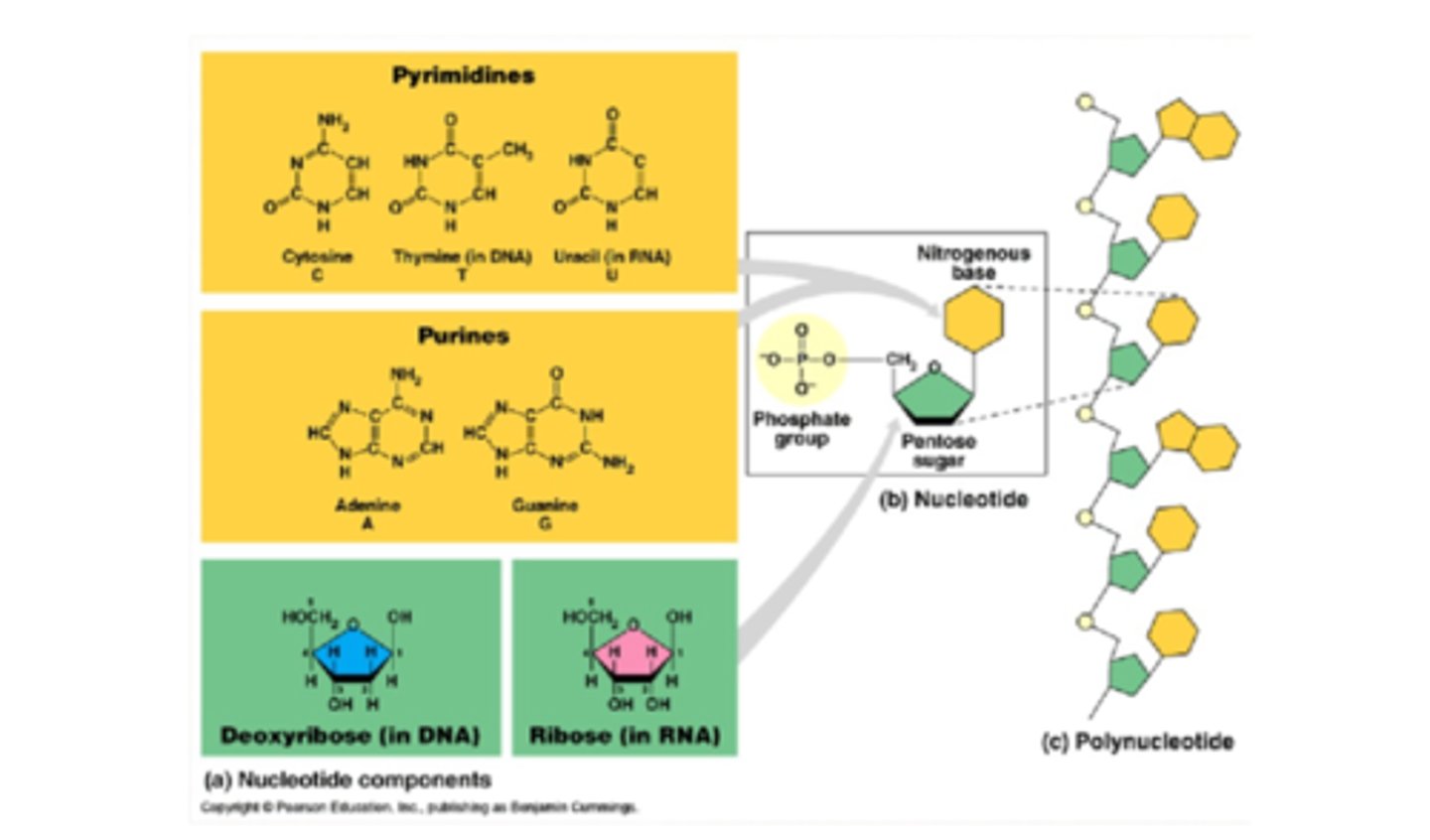
What makes up nucleic acids?
- CHONP
- composed of:
- pentose sugar (ribose or deoxyribose)
- nitrogenous base
- phosphate group
- nucleotide = nucleoside (sugar and base) + phosphate group
What are the purine bases?
- adenine
- guanine
*double ring structure
What are the pyrimidine bases?
cytosine, thymine, uracil
*single ring structure
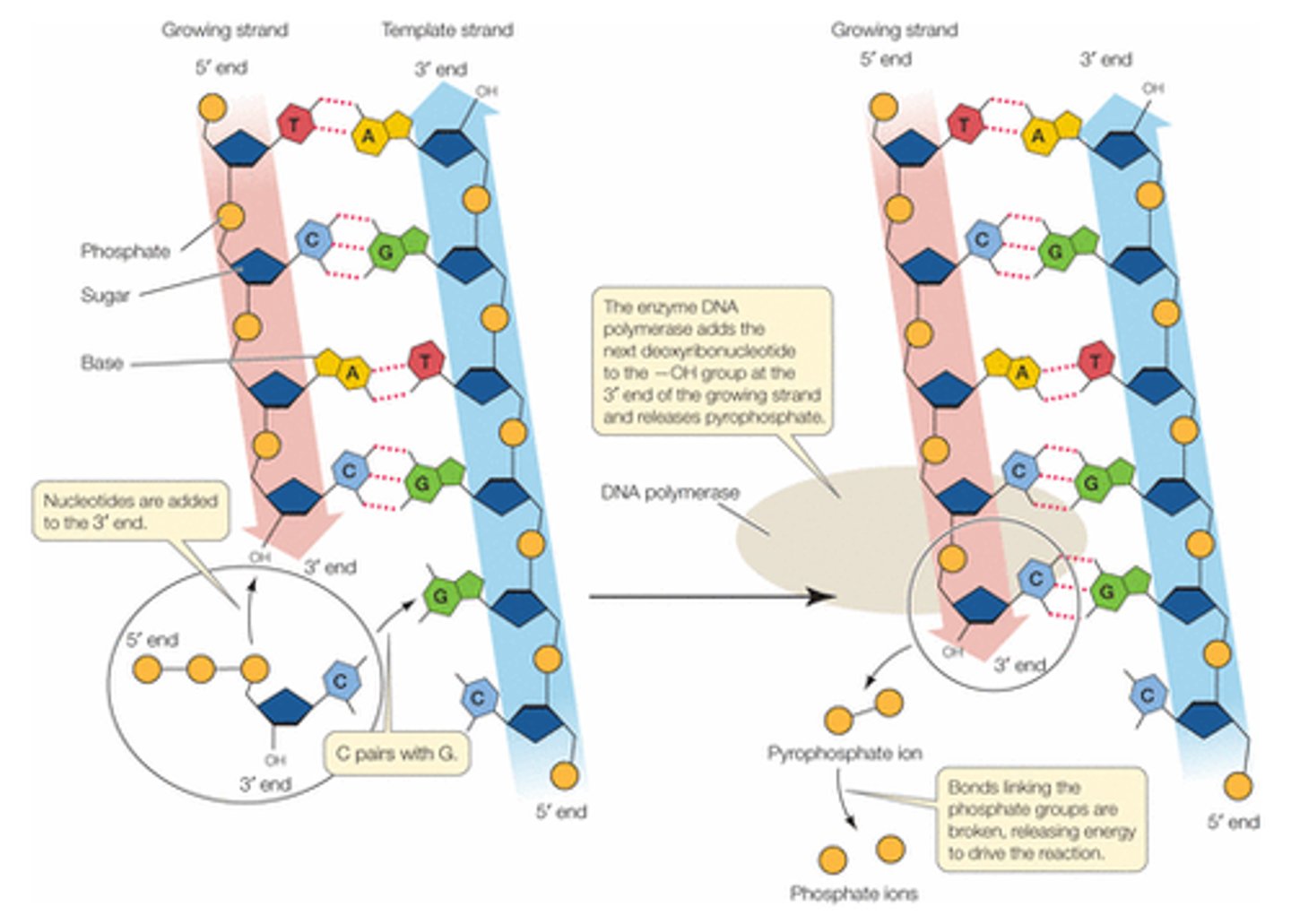
What are the two types of bonds involved in DNA?
- phosphodiester bonds: in between 2 adjacent bases
- hydrogen bonds: in between complementary bases
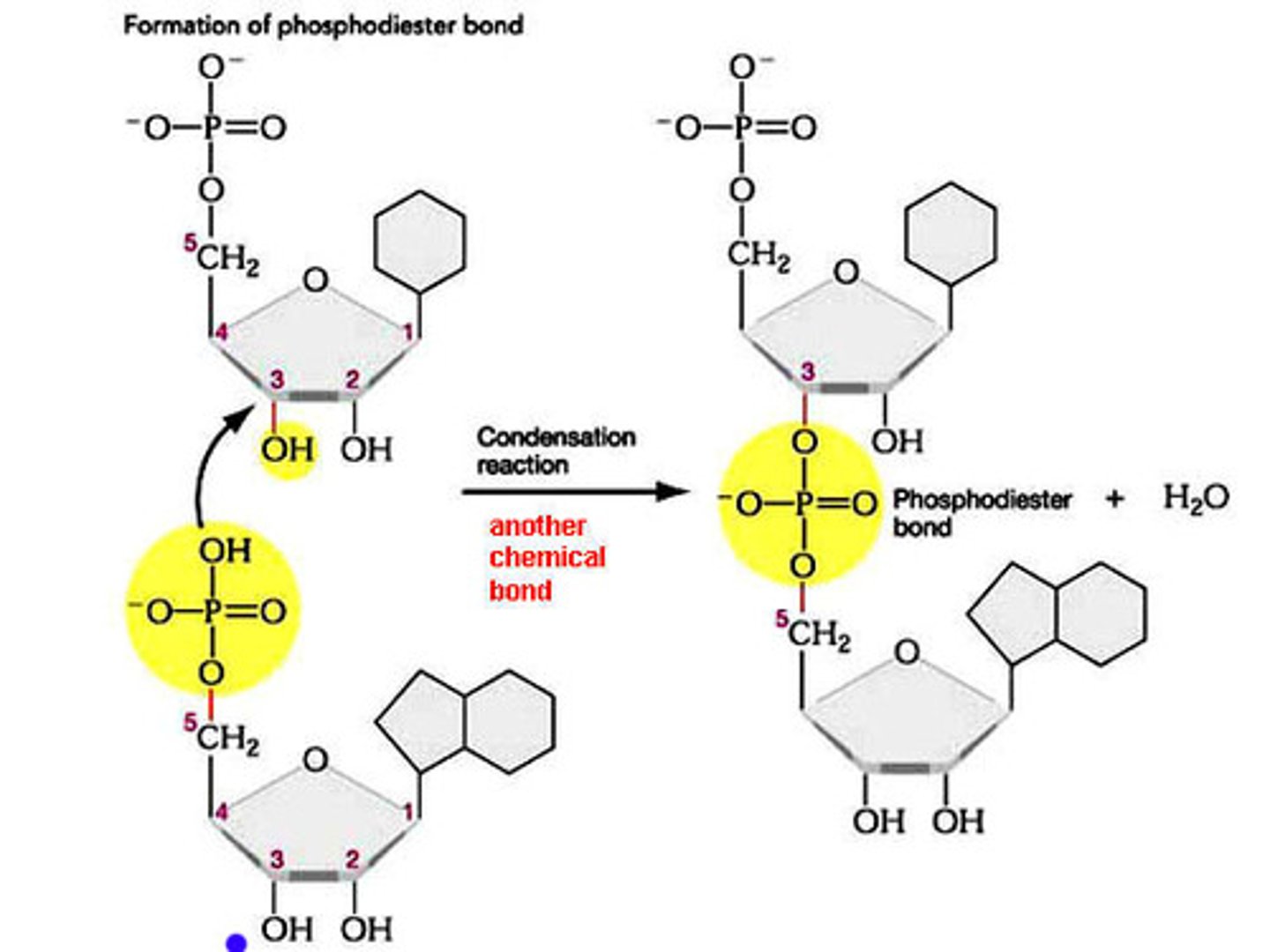
What are phosphodiester bonds?
- phosphate-sugar backbone of DNA
- condensation reaction (water is produced)
- phosphate on 5' carbon reacts with 3' carbon, imparting directionality
Describe hydrogen bonds in DNA
- in between complementary bases
- A-T = 2 hydrogen bonds
- G-C = 3 hydrogen bonds
What is the primary structure of a protein?
sequence of amino acids
What are amino acids linked by?
peptide bonds
- covalent bonds formed between carboxylic acid and adjacent amine group
How is the polypeptide chain extended?
- amino acids are brought together by covalent bonds to form the polypeptide backbone
- N-terminus: the end of the protein sequence that is an amine group
- C-terminus: the end of the protein sequence that is a carboxyl group
What is the secondary structure of a protein?
- noncovalent hydrogen bonds form with the peptide backbone and side chain interaction
- commonly formed structure from H-bonding of the peptide backbone: alpha helices and beta sheets
- polypeptide chains ultimately fold into the lowest energy state conformation
- folding of the polypeptide chain confers a specific activity through active regions, binding sites, and root structure
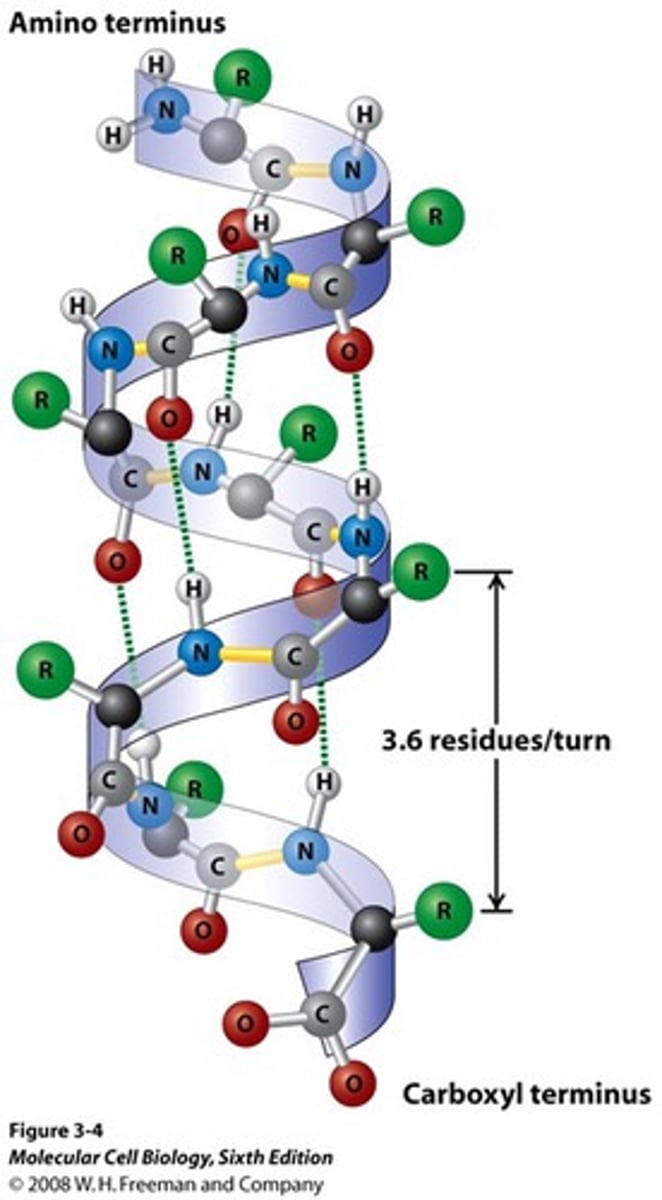
What are alpha helices?
- initially identified in hair, though present in derivatives such as skin, horns, nails
- An alpha-helix is generated when a single polypeptide chain turns around itself to forma. structurally rigid cylinder; directionally, they can be right or left handed (strong bias to R)
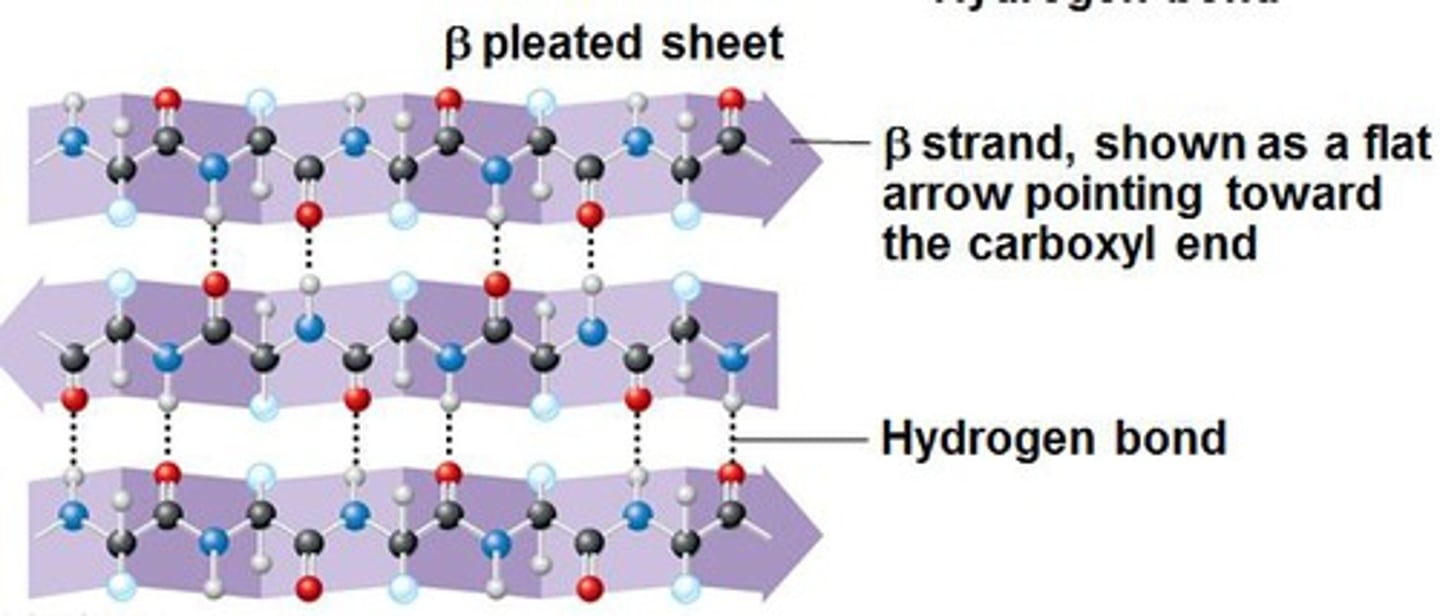
What are beta sheets?
- formed when hydrogen bonds form between segments of polypeptide chain that lie side to side
- the projection of residues above and below the polypeptide backbone allows the association of parallel/anti-parallel chains through hydrogen bonding
- the rigid, pleated structure that results affords remarkable tensile properties (ex. silk), in addition to anti-freeze properties preventing ice development on cold weather beetles
What is the tertiary structure of a protein?
The final 3D structure of the folded polypeptide chain: the sum of the beta sheets, alpha helices, hydrophobic regions, polar interactions that contribute to a functional protein
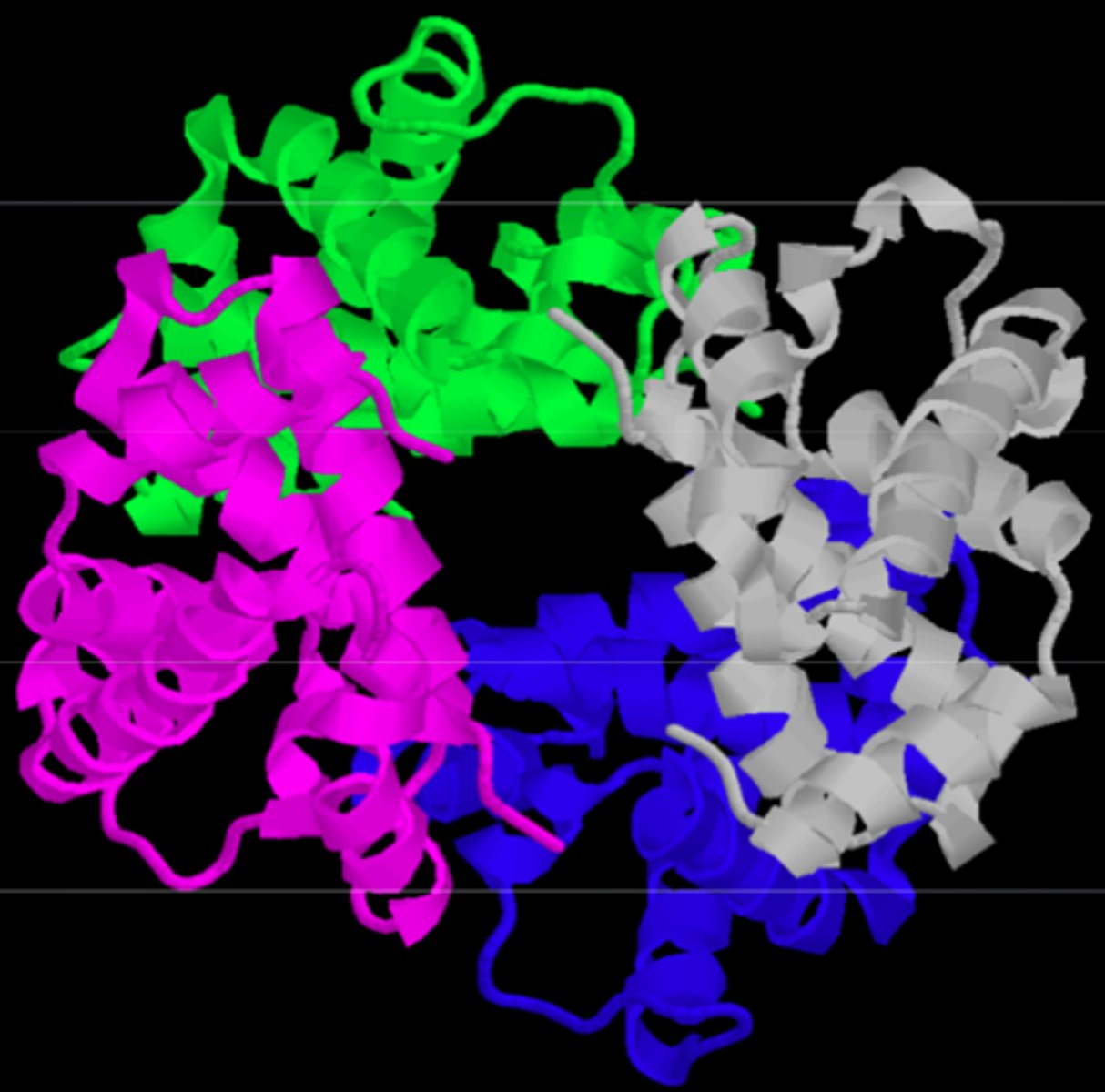
What is the quaternary structure of a protein?
- final functional form of a protein: the interaction of multiple subunits
- the final protein may be a repeat of a single polypeptide subunit or an assembly of different subunits
What is the importance of non covalent bonds in protein folding?
- help proteins fold and maintain their shape → crucial to function
- they are weaker than covalent conds but their energies can sum to create an effective force between two molecules
- the stability of each folded shape is largely influenced by the combined strength of large numbers of noncovalent bonds
- each protein normally folds into a single stable conformation which can change slightly when the protein interacts with other molecules in the cell
What are the types of non covalent bonds?
- hydrogen bonds: formed when a hydrogen atom attached to an electronegative species (O or N) is in proximity to another extronegative species
- electrostatic attractions: When a molecule with many polar groups and a pattern of partial positive and negative charges on its surface encounters a second molecule with a complementary set of charges
- van der Waals attractions: a form of electrical attraction caused by fluctuating electric charges that arise whenever two atoms come within a very short distance of each other
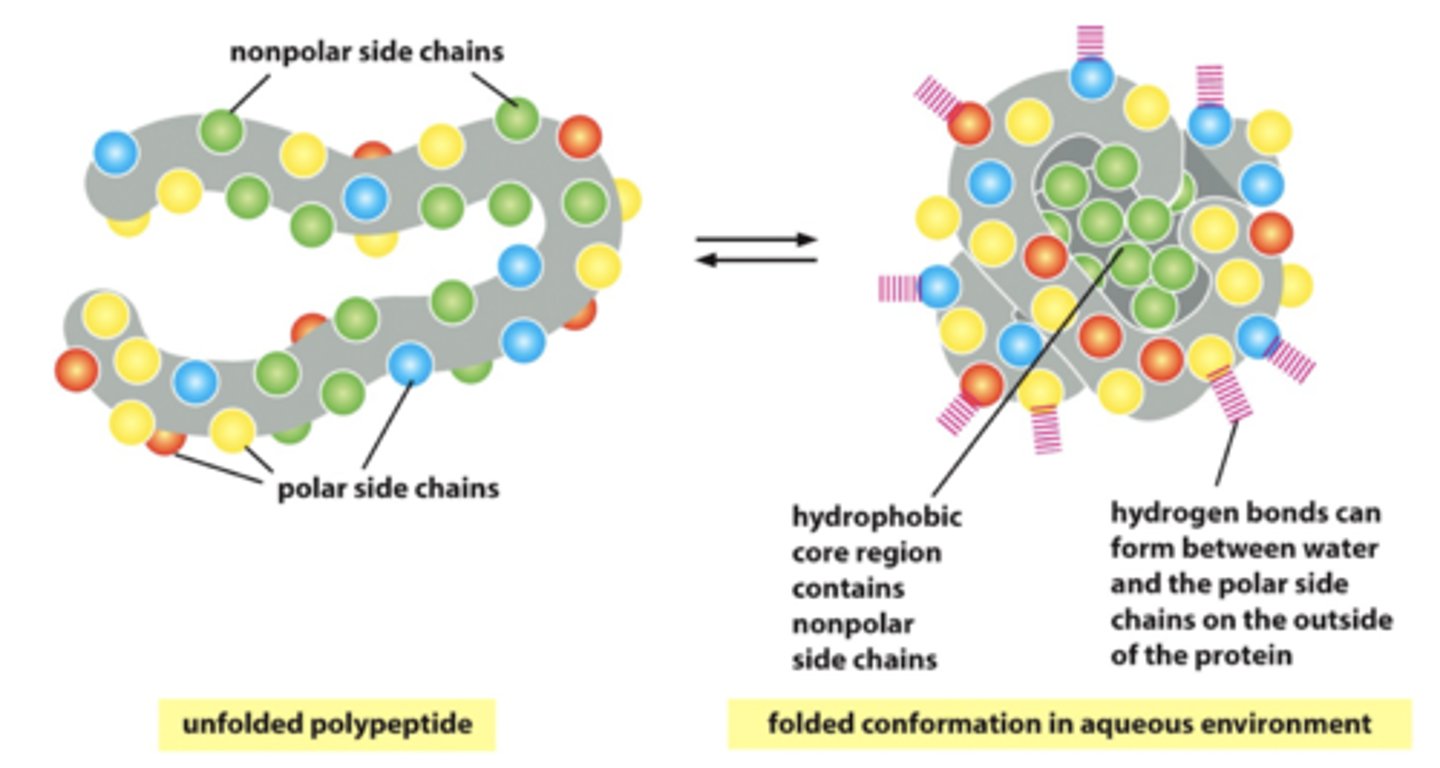
What are hydrophobic interactions?
the clustering of non-polar or hydrophobic side chains in the interior of the folded protein to avoid contact with aqueous cytosol
What do higher levels of protein organization look like?
- seen as filaments, spheres, and tubes in a repeatbale pattern
- fibrous proteins maintain a relatively simple, elongated structure to fit role in ECM and intracellular reinforcement
What is disulfide bonding?
- oxidation of the sulfhydryl groups on cysteine allows for different parts of the protein chain to be held together covalently
- contributes to a more stable tertiary structure
- stabilization of ECM can incorporate disulfide bonds-covalent bonds that form between spatially adjacent cysteine side chains; serving as reinforcement within or between chains
What is the role of Ammonium Thioglycolate in a hair perm?
- reduces disulfide binds in the cortex of the hair. After washing, the hair is treated with a mild solution of hydrogen peroxide, which causes new bonds to form, giving hair the rigid structure necessary for permanent waves
- reducing agent: ammonium thioglycolate
What is mad cow disease an example of?
what happens when proteins do not fold- function is hindered
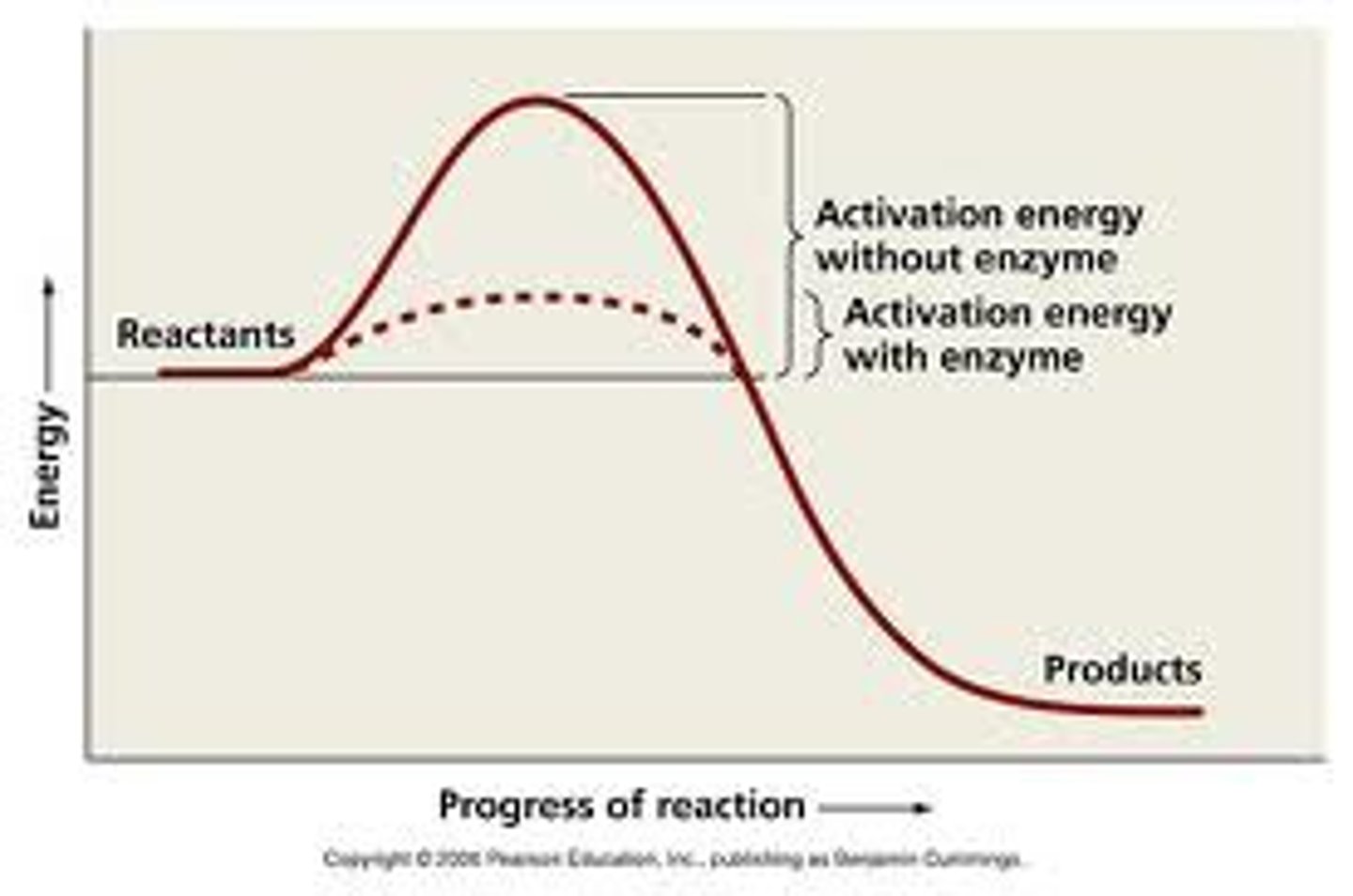
What are enzymes?
help catalyze reactions; able to speed up energetically favorable reactions, but cannot force energetically unfavorable ones to occur
- frequently targeted for drug inhibition due to aberrant regulation-inactivity or over-activity
- They physically bind to a specific substrate
- convert substrates to products while remaining
unchanged themselves
- binding initiates their downstream function- bond formation, cleavage- in a specific and ordered fashion
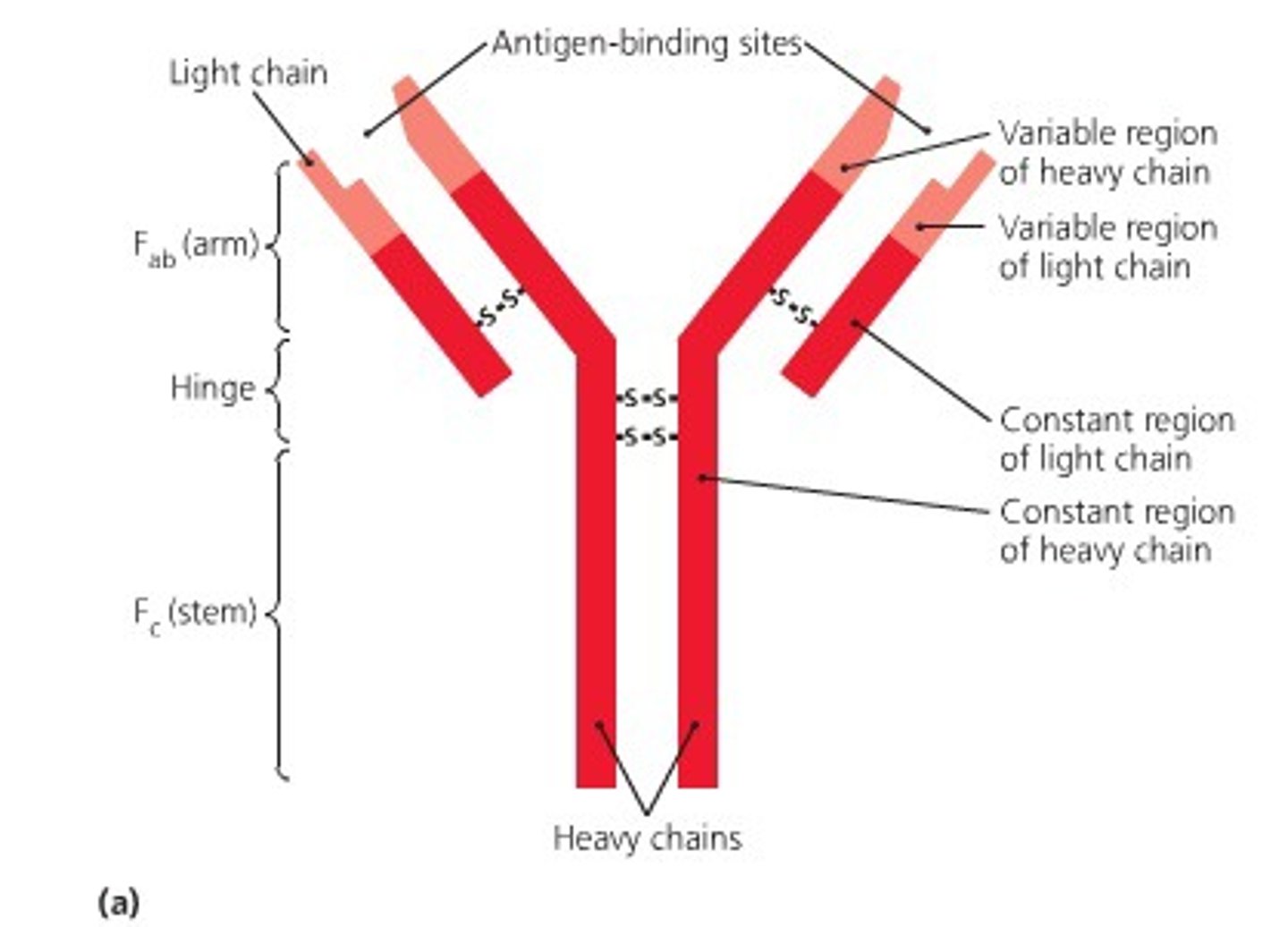
What are antibodies?
- generated by the immune system: immunoglobulin proteins
- recognizes a specific target molecule or antigen with high specificity
- have two heavy chains and 2 lights chains: variable regions in the light and heavy chains accommodate different binding sites to target different regions of foreign bodies/target proteins
- Produced by B Cells that that individually all have different binding regions presented; when an antigen binds, the cell rapidly divides and secrete soluble antibodies that target the antigen to be engulfed or destroyed
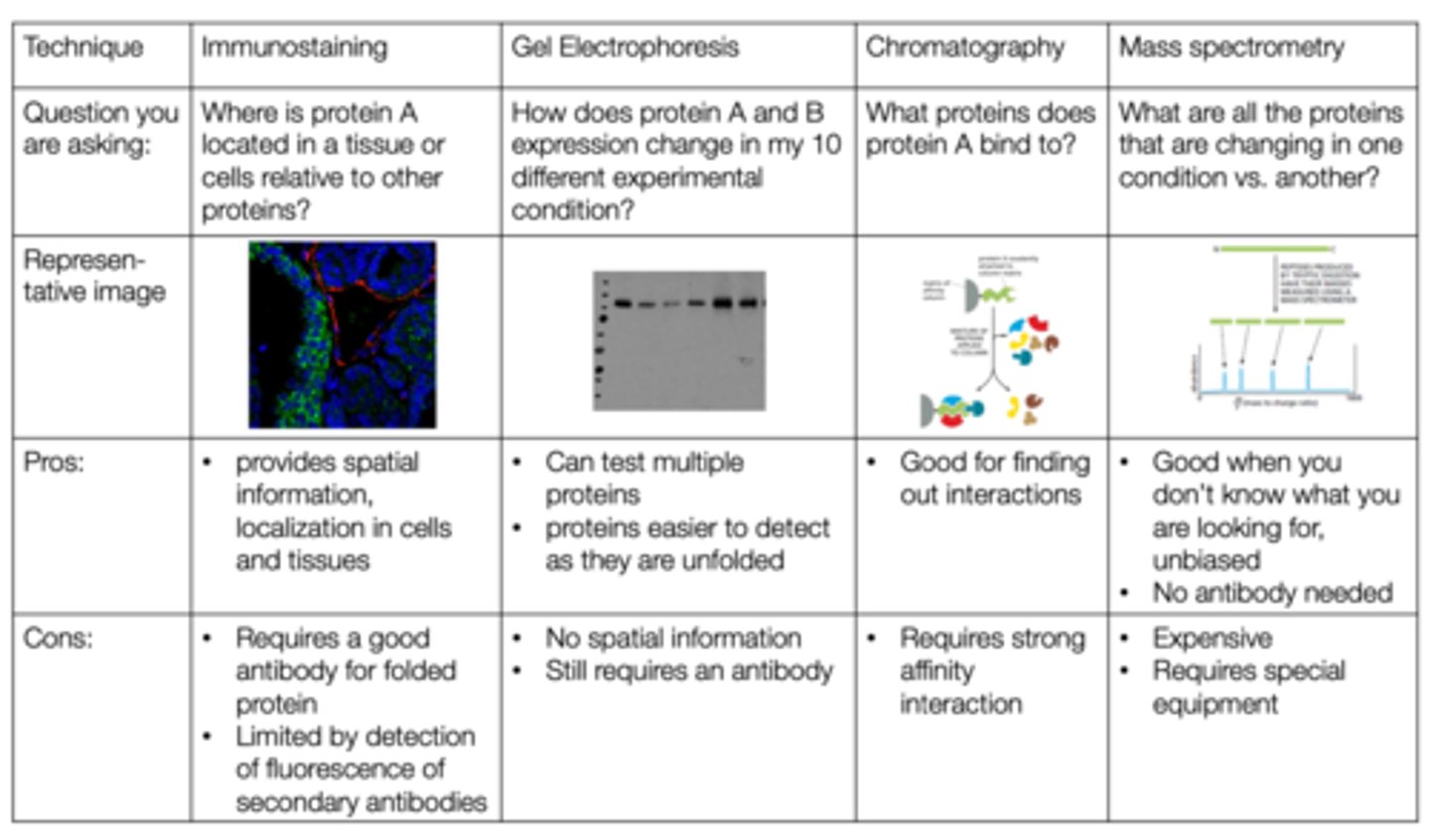
What are the methods of studying proteins?
- gel electrophoresis/ western blot
- immunostaining
- chromatography
- mass spectrophotometry
What is western blot?
Electrophoresis:
1. Cell lysis: breaks down cells to isolate proteins.
Mercaptoehtanol treatment breaks disulfide
linkages to denature protein and have it unfolded.
2. Protein separation on a gel:sodium dodecyl
sulfate added to give uniform negative charge to
proteins and current puls protein down get to
positive chargeProteins are separated based on
size: big at the top, small at the botton
Protein Detection: use antibodies to detect proteins at specific molecular weight
- protein size is measured in kilodaltons
→ Pros: can test multiple proteins, proteins easier to detect by antibody as unfolded
→ Cons: No spatial info
What is immunostaining?
- antibody-based method to detect a specific protein in a sample
- fluorescently tagged secondary antibody against species of primary, primary antibody against protein of interest
- antigen: AA sequence specific to that protein to be recognized by the antibody
Pros: provides spatial info, localization in cells and tissues
Cons: Requires an antibody that can recognize the sequence with the folded structure, limited by detection of fluorescence of secondary antibodies
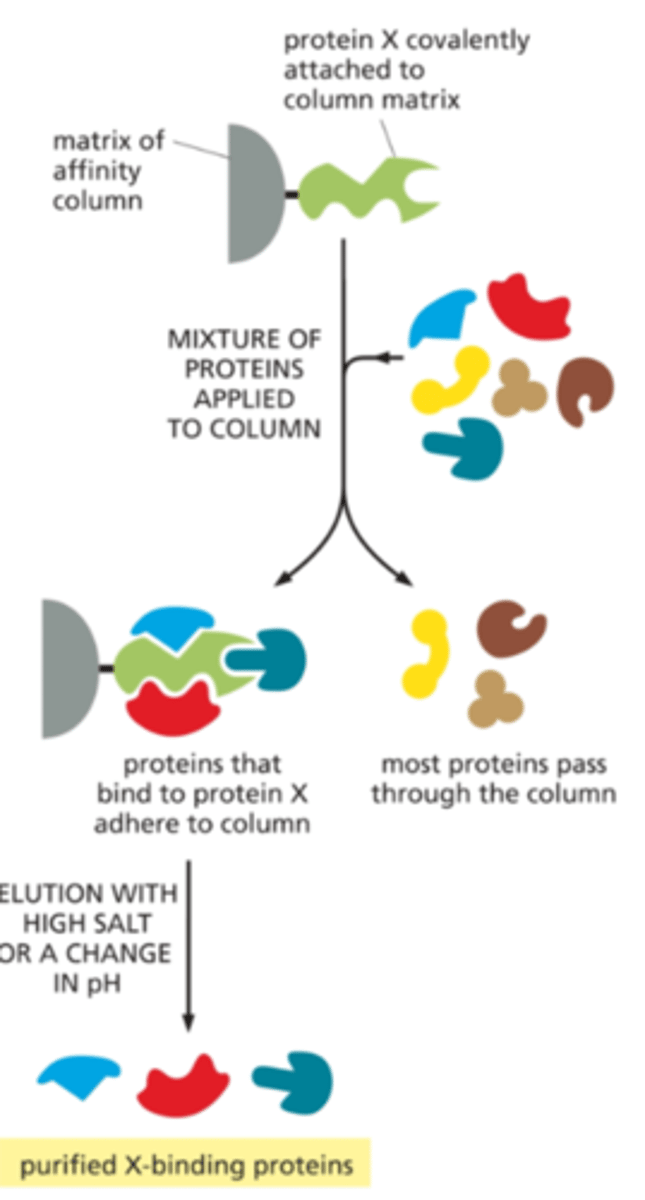
What is chromatography?
- isolate binding partners of a protein of interest
- Purified protein of interest is attached tightly to the coumn matrix
Pros: good for finding out iteractions
Cons: Requires strong affinity interaction
What is mass spectrophotometry?
- proteins are digested into smaller pieces using trypsin (cleaves polypeptide chains on the carboxyl side of a lysine or an arginine)
- laser heats peptides, cause them to become electrically charged, and ejected as a gas
- Iions fly by detector, which records time it takes for the to arrive, which is related to mass and charge
- can be applied to protein mixtures
Pros: Unbiased, can look at many proteins at once
Cons: Expensive, no localization info
"Mass spectrometry is an important method for the accurate mass determination and characterization of proteins, and a variety of methods and instrumentations have been developed for its many uses"
Describe the DNA backbone
- composed of nucleotides brought together by a covalent phosphodiester bond
- nucleotide is composed of a phosphate group, pentose sugar, and nitrogenous base
- base pairing occurs joining complementary strands of DNA by hydrogen bonds
How many hydrogen bonds between A-T?
2 hydrogen bonds
How many hydrogen bonds between G-C?
3 hydrogen bonds
Describe the directionality of DNA
- carbons numbering: 1' carbon is the one with the base, then go clockwise
- phosphate on 5' carbon reacts with 3' carbon
- reads up (3→5), writes down (5→3) because it is ANTIPARALLEL
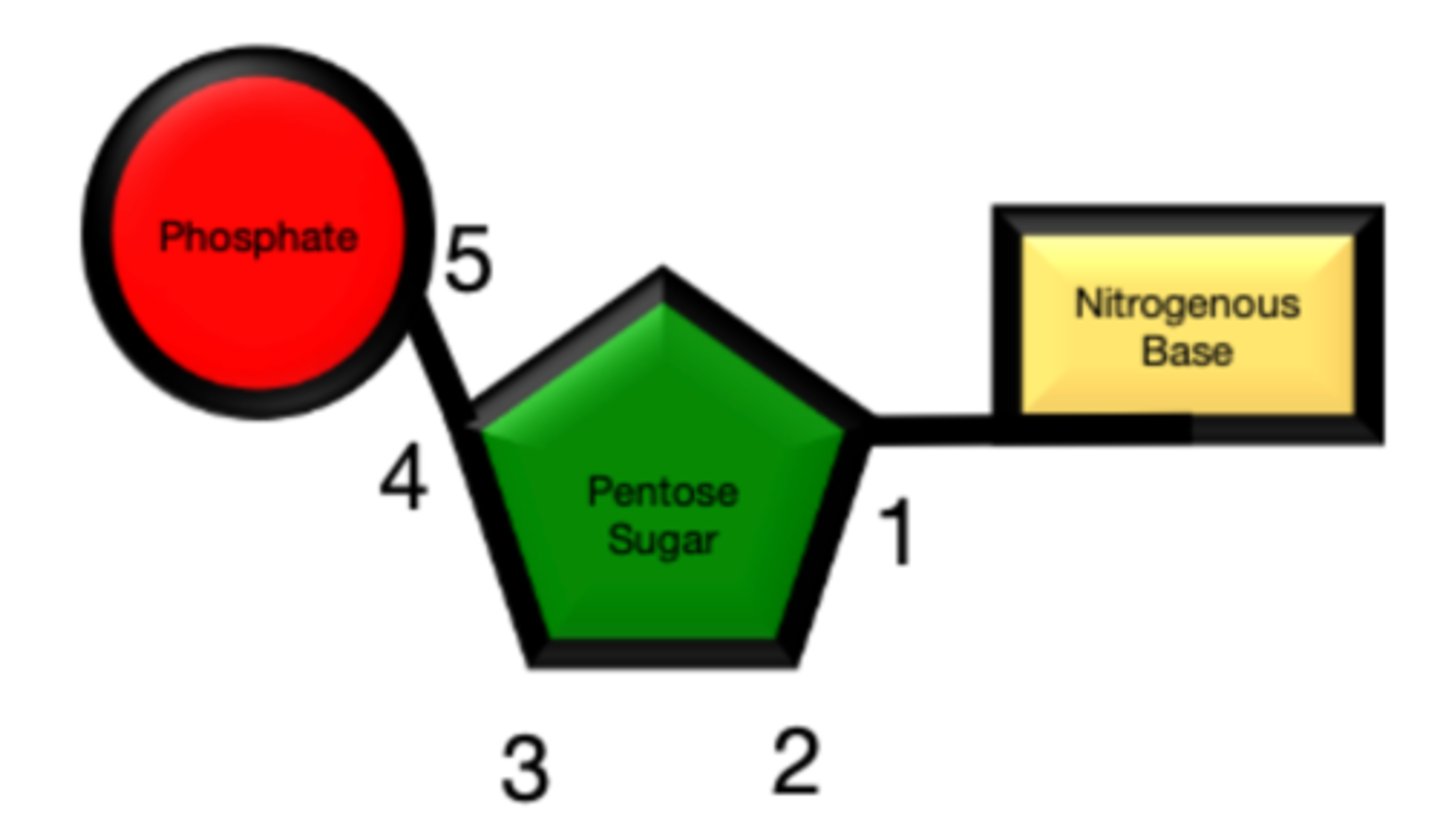
What is the charge of DNA?
- DNA backbone is negatively charged due to the phosphate groups
The DNA double helix is ___ handed
- double strand DNA is a right handed helix
- the 2 helices runs antiparallel to each other
What genetic information is found in each somatic cell?
- There is 6ft of DNA in a cell nucelus of 5 micrometer in diameter
- 22 chromosome pairs- representing one from each parent- and 2 sex chromosomes
What represents the human karyotype?
display of the full 46 chromosomes
What are some issues involving chromosome number?
- genetic defects occur due to abnormal chromosome structure or number
- some disorders are caused by deletion of
chromosomes
- down's syndrome (trisomy 21)- median of 49 years
- Edward's syndrome (trisomy 18)- days
- patau's syndrome (trisomy 13)-months
What does it mean to have two copies of each chromosome?
2 copies of each gene
allele: variant form of a given gene at gene locus
homozygous: both alleles are the same
heterozygous: alleles are different
What is the structure of chromosomes like?
- Telomeres: located at the end of a chromosome, these regions contain repeated nucleotide sequences that enable the ends to be replicated
- Centromeres: dense, central region of a chromosome that anchors the pairs for separation during mitosis
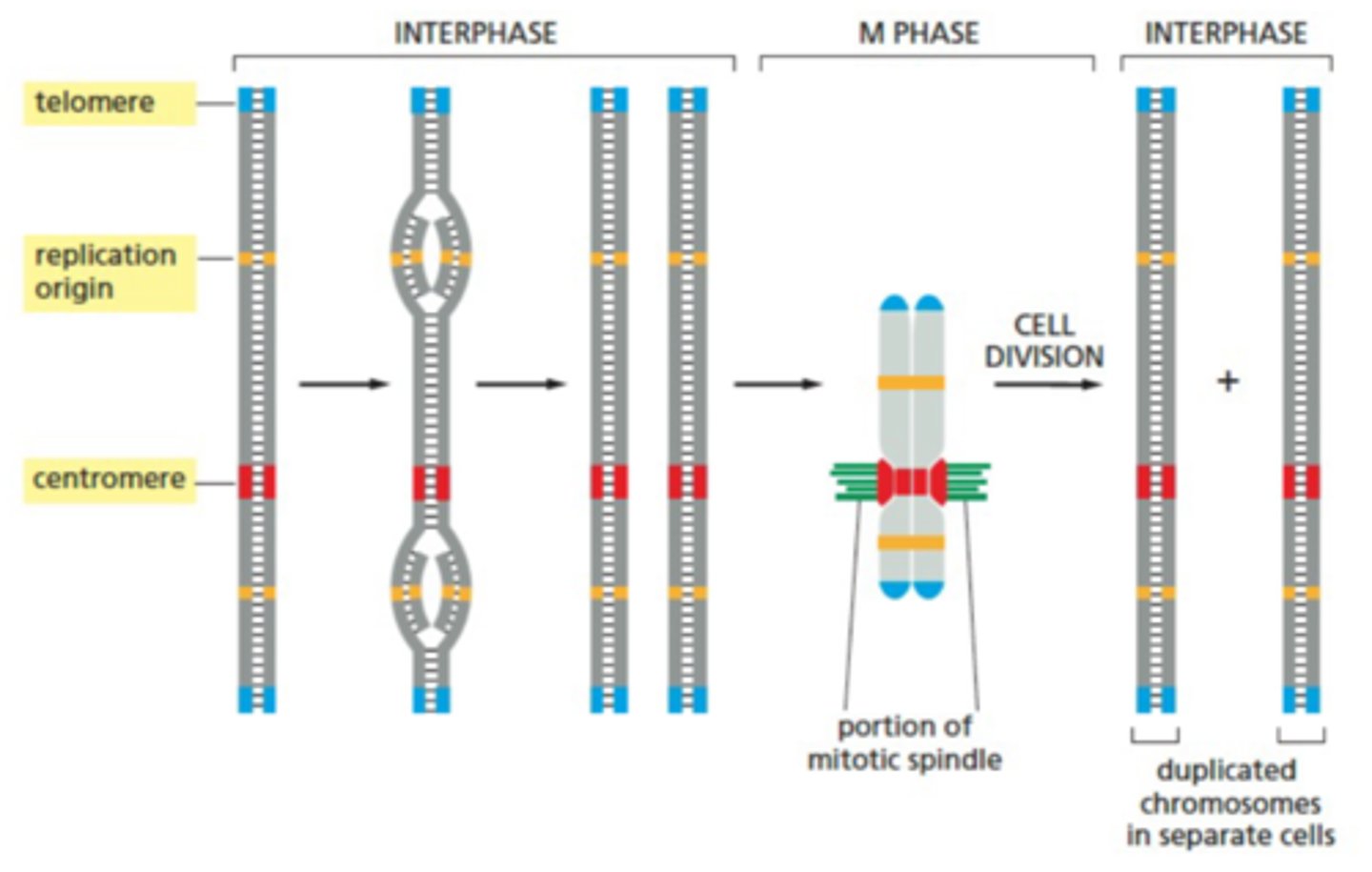
How is DNA so tightly packed into chromosomes?
- Interphase nuclei were lysed and the resulting chromosomes imaged
(A) Chromatin isolated from an interphase nucleus as 30nm thick chromatin fiber
(B) Partial nuclease digestion revealed “beads on a string”- chromatin wrapped around histone cores that were termed nucleosomes
What are histones?
- histones are a class of DNA binding proteins (vs nonhistones) and are one of the most highly conserved genetic sequences
- relatively small with high proportion of positively charged AAs (lysine and arginine) helping to associate the negatively charged backbone of DNA
- the functional unit is an octamer, binding 147 bps around the assembled core. Each octamer consists of 2 units each of H@A, H2B, H3, and H4
- nucleosome is full core plus associated linker DNA
- histones has a more positive charge which allows for a tight attraction between DNA and histones