Proteins SL and HL
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
proteins
fiberous proteins
offer strength and support structurally, Fibrous proteins consist of elongated polypeptide chains that lack typical tertiary structures. Instead, their quaternary structure involves linking polypeptides into long fibres or filaments, stabilized by hydrogen bonds.
globular proteins
Globular proteins are compact, spherical proteins that are typically soluble in water. Their structure results from the folding of the polypeptide chain into a tightly packed three-dimensional shape. Globular proteins have a rounded shape, formed by the folding of polypeptides into intricate structures. These shapes are stabilized by bonds between R-groups.
example of globular protein
Conformation: Insulin’s specific three-dimensional shape allows it to bind precisely to insulin receptors on cells.
Function: This precise binding triggers intracellular signals for glucose absorption, essential for maintaining blood sugar levels.
Functionality
The precise arrangement of atoms, known as conformation, is critical for the function of globular proteins. Examples include:
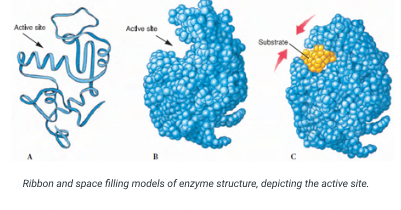
Enzymes: Their active sites rely on the precise folding of R-groups to catalyze reactions.
Receptors: Ligand-binding sites depend on specific protein conformations to transmit signals.
genetic code connection
it’s universal so the 20 amino acids are encoded by the same genetic code across all species, the code is read as three-nucleotide mRNA codons, each coding for one specifc amino acid
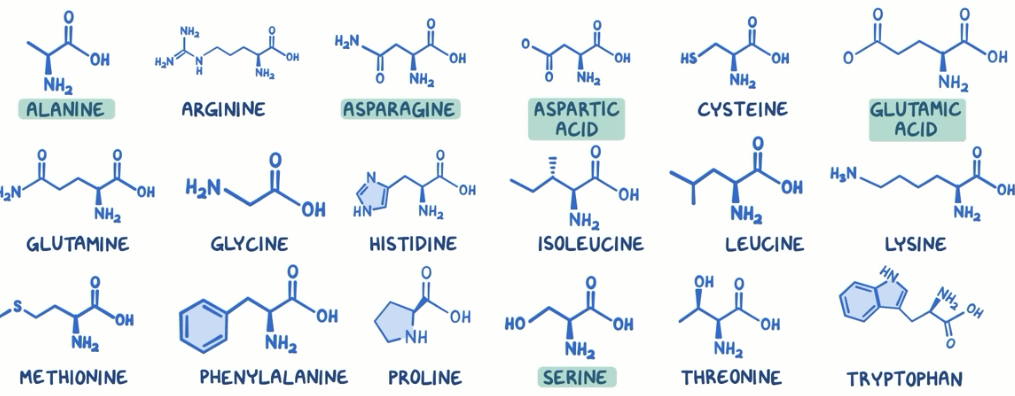
amino acids
monomer of proteins
oligopeptides
Short chains of 3-20 amino acids
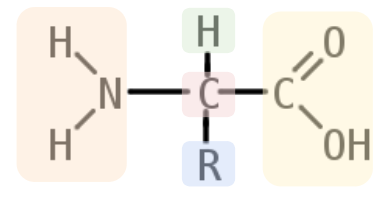
basic structure of amino acid
amine group, alpha carbon(backbone sturcture in polypeptides), hydrogen atom, variable side chain, carboxyl group
non-essential amino acids
5 types of amino acids we can get fromfood and make ourselves
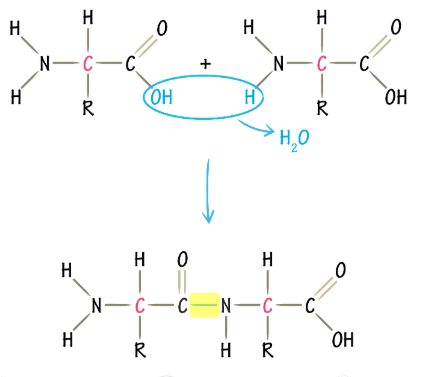
dipeptide
two amino acids joined via a condensation reaction removing one molecule of water and forming a peptide bond(C-N)
conditionally essential amino acids
6 amino acids our body can make if healthy eg. can’t make when starving or with inborn error of metabolism
variety in amino acids
The R-groups are the functional gorups makeing the 20 amino acids different(some polar, non polar, charged and uncharged, acidic or basic, some ringed structured and variety of elements) and this composition is going to affect how amino acids in a polypeptide interact with each other, affecting the folding, and the particular sequence of amino acids is determined by the DNA.
protium
set of proteins we can make in an organism, we can make and infiite number of possible coded for by our DNA
condensation reactions/ dehydration synthesis
builds complex molecules by joining two or more molecules together, removing a molecule of water, it’s an anabolic process requiring enrgy to occur and catalyzed by enzymes, forms a peptide bond between carboxylic group and amine gorup of two amino acids.
location of polypeptide synthesis
occurs in the ribosomes which are repsonsible for translating mRNA into popylpeptides
hydrolysis
amylase
Enzyme in saliva that breaks down starch, Composed of a single polypeptide chain with 496 amino acids§§§
collagen
Quaternary Structure: Composed of three polypeptides twisted into a triple helix.
Primary Structure: Features a repeating sequence of three amino acids, proline, glycine, and X (variable).
Glycine, with its small R-group (a single hydrogen atom), faces inward, allowing the tight triple helix structure.
Proline or hydroxyproline prevents the formation of alpha-helices, facilitating the triple helix.
Functionality
Tensile Strength: Collagen’s rope-like triple helix provides structural integrity to tissues such as skin, tendons, ligaments, and cartilage.
Variability: The R-group of the third amino acid is flexible, enabling different types of collagen for various functions (e.g., forming the basement membranes of epithelia and the outer layer of the eye).
haemoglobin
denaturation
process by which a protein unfolds from its functional structure due to the disruption of the interactions that stabilise protein structure,
including hydrogen bonds, ionic and hydrophobic interactions which when overcome by environmental stresses the protein unfolds into a more random and extended conformation losing its 3D arrangement of amino acids required for biological activity is destroyed.
It’s often a permanent chnage but sometimes can renature. eg. render a protein that used to be soluble into insoluble
temperature effect on proteins
kinetic energy and molecular motion increases with temperature, causing greater and faster vibration and movement of atoms within protein molecules.
metabolic protein function increases with small increases in temperature because the greater molecular motion leads to more frequent and energetic collisions between proteins involved in biochemcial reactions
pH effect on proteins
Each protein has an optimal pH range within which it functions, and if it is placed in environments outside of this range, the protein can break bonds/interactions, unfolding its shape and shifting its function.
As pH chnages the charge of amino acid R-groups can change altering the pattern of electrostatic interactions in the tertiary structure and causing protein denaturation
at low pH, negatively charged amino acid R groups become protonated changing their negative charge to neutral
as pH increases and the solution becomes more basic positively charged amino acid R groups(HIS, LYS and ARG) can lose a proton(N- becomes N) changing their positive charge to neutral. These electrostatic interactions between charged R-groups contribites significantly to protein stability, and can influence protein to protein interactiosn and enzyme-substrate binding.

protein conformation
the specific 3D shape of a protein molecule, the native conformation is it’s most stable and natural shape
insulin
Hormone that regulates blood sugar levels and Made of two polypeptide chains, one with 21 amino acids and the other with 30.
polar interactions in tertiary structure
polar- partially positive and partial negative charge due to unequal electron sharing between atoms in the R groups, easily form hydrogen bonds with other polar molecules, such interactions are crucial for protein folding, enzyme specificity and protein-protein interactions that drive cellular processes.
hydrophobic and hydrophylic interactions
hydrophobic- amino acids with R groups composed of primarily of carbon and hydrogen atoms are nonpolar and hydrophobic, these tend to minimize their contact with wayer typically clustering together in the interior of globular proteins , away from the aqueous environment
hyrdophylic- interact favourably with water through hydrogen bonding or electrostatic interactions, often appear on protein surfaces interact with the aqueos envirnoment which enables proteins to maintain solubility in cellular environments and facilitates interactiosn with other polar molecules including proteins and nucleic acids.
example of polar interactiosn in enzymes 3D structure
Enzymes’ active sites are typically located in pockets on the protein surface and the active site containes a mixture of polar and nonpolar amino acids that create a chemical environment that is specific to the particular enzyme substrate whiche nsures that only chemcially complementery substrates are able to bind to the enzyme.
ionic bonds in 3D structure
positively charged R groups: have a nitrogen atom that can gain a hydrogen to become N+ and negatively charged R groups: have a carboxyl group that can lose an H atom to become COO-, they for ionic bonds with oppositely charged amino acids.
amphipathic
hydrophobic and hydrophylic regions
integral membrane protein
integral membrane proteins permanently embedded withina plasma membrane with roles in transporting molecules, transmiting chemcial signals, and facilitating cell adhesion, integral membrane proteins are amphipathic. Nonpolar hydrophobic amino acids willa rrange themselves within the hydrophobic core of the bilayer with the fatty acid chains of phospholipids.
quaternary structure
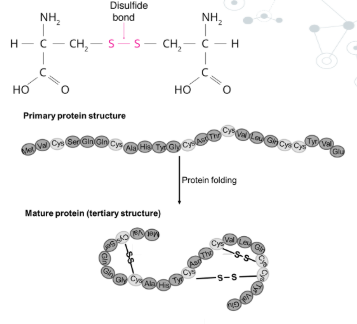
disulfide bridges
disulfide covalent bonds between cysteine amino acids that are close together in the primary sequence or between cysteine close together in the folded structure to cause further folding, they are the most stronf bonds in tertiary structure emhancing stability in proteins exposed to harsh conditions.
polar
has a positive and negative charged region, hydrophylic and struggle to difuse across cell membrane
non polar
electrical charged evenly distributed(no negative or positive region), nydrophobic and easily diffuses acrss cell membrane.
essential amino acids
our bodies can’t produce them and thus we msut get them from food, 9 types - histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine
complete proteins
Contain all nine essential amino acids in proportions similar to what the human body needs. eg. eggs, meat, fish
incomplete
Lack one or more essential amino acids but contain some of them, in different proportions, eg. Cereals (e.g., wheat): Low in lysine and Legumes (e.g., beans and peas): Low in methionine. Combining lots of different plant foods can profide a compelte amino acid profile.
vegan diet concerns
must be very purposeful and mindful about what protein sources confirmed as they all containe some essential amino acids but not in the same proportions as the body needs them and lack some, thus by combining plant foods with complimenting amino acid profiles form very varied sources vegans can still meet the needs.
eg. meal that is complimentary for vegans
rice- Low in lysine but high in methionine.
beans-Low in methionine but high in lysine.
Rice and beans create a meal that provides all essential amino acids in sufficient quantities.
risks of deficiencies in certain amino acids
muscle wasting: skeletal muscles are digested to release amino acids for more critical organs and functions, decrease in muscle mass
stubnted growth in children
reduced immune function making one suseptible to illness and slowing recovery and healing process
Feeling more tired and lethargy
Deficiencies in certain amino acis can affect certain neurotransmitter production and potentially contrute to depression, anxiety and iritability.
Hair loss, thininning of hear and skin problems as they are heavily dependent on protein production.
why protein consumption matters
Proteins are essential for nearly every biological process in your body, from building muscle to producing enzymes and hormones. A deficiency in essential amino acids can disrupt these processes, leading to a cascade of health issues. Understanding amino acid requirements helps maintain overall health and biological functions.
number of possible combinations with “n” amino acids
Tripeptide (3 amino acids): 20 to the power of “n”
20 to the power of 3= 8,000 possible combinations.
non-conjugated
Made entirely of polypeptide subunits
Examples include insulin (two chains linked by disulfide bonds) and collagen (three chains in triple helix) 1
Function depends only on the arrangement and interactions of polypeptide chains_
conjugated
Contain polypeptide chains plus non-polypeptide components called prosthetic groups
Example: hemoglobin has four polypeptide chains, each with a heme group containing iron (Fe²⁺) 2
The non-polypeptide components are essential for biological function (heme groups bind oxygen)
conjugated
diversity in proteins
While natural constraints exist, there is an infinite length and combinations of amino acids theoretically. Changing just one amino acid in a sequence can dramatically alter the protein folding and thus stability and function.
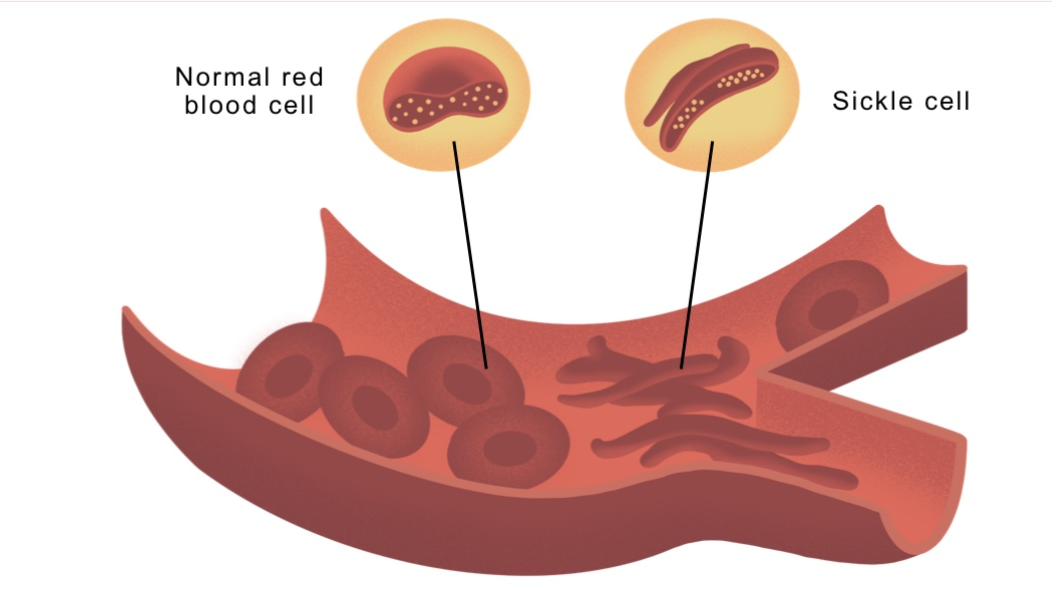
sickel cell anemia
example of how chang in one amino acid can alter conformation of a protein, it’s caused by a single nucleotide mutation in the haemoglobin HBB gene and this adenine to thymine substiution chnages an amino acids of haemoglobin protein from glutanine to a valine and this valine amino acid is hydrophobic and causes the molecule to form long, inflexible chnages that lead to stiffening of the red blood cell causing it to have the sickle shpae
primary structure
linear sequence of amino acids joined together by peptide bonds, these covalent bonds are relatively stable under typical physiological conditions
provide the permanent structural framework of a protein and the sequence is determined by the genetic code.
The primary structure of a protein determines the final confromation of a protein
secondary structure
regular repating alpha helices and beta pleats that form in polypeptide chains through hydrogen bonding between atoms in the polypeptide backbone(nitrogen, alpha-carbon, and carbonyl atoms formed when the amino acids link by peptide bonds).
alpha helix is a spiral where the polypeptide backbone coils around an imaginary central axis, hydrogen bonds form between atoms od the polypeptide backbone roughly every 4 amino acids.
beta pleated sheets structure forms hen a polypeptide chain folds back on itself and amino acids align side-by-side and form hydrogen bonds between their backbones.
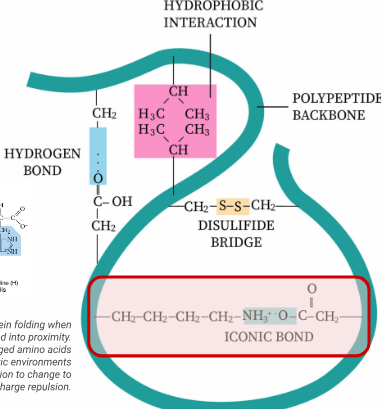
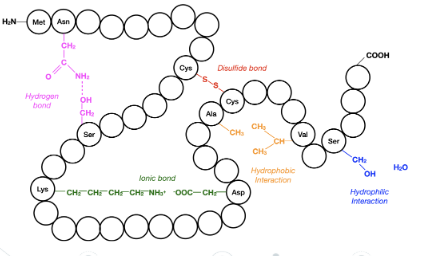
tertiary
the overall 3D folding pattern of a complete polypeptide chain determined by interactions between amino acid R-groups of the polypeptide chian, deciding it’s function. Molecular forces between R groups include:
hydrogen bonds between polar R groups
ionic bonds between charged R gorups
disulfide covalent bonds/bridges between cyteine amino acids
hydrophobic interactions between nonpolar R-groups
quaternary
Multiple polypeptide chains assembled together
Each chain is called a subunit
Example: Insulin consists of two chains (21 and 30 amino acids