Nervous system
0.0(0)
Card Sorting
1/113
Earn XP
Description and Tags
Last updated 9:22 PM on 1/9/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
114 Terms
1
New cards
Action potential
Rapid, reversible change in membrane potential, consisting of a depolarisation follow by a repolarisation.
2
New cards
Model organism/species
Squid!!! They have a giant axon (1mm diameter) used by Hodgkin and Huxley.
3
New cards
Voltage clamp
Electronic device that allows measurement of whole-cell ionic currents. Operates by setting the membrane potential to a predetermined value and measuring the charge needed to get there.
4
New cards
Nerve cell resting potential
\-70mV
5
New cards
Properties of action potential
Voltage-dependent and all-or-none
6
New cards
Uncoupling membrane potential and ion permability
Voltage clamp function, in order to measure cell current
7
New cards
Rising phase/depolarisation (AP)
Increasing membrane permability to Na+. The overshoot heads towards ENa.
8
New cards
Repolarisation (AP)
Na permeability falls, K permeability rises.
9
New cards
Hyperpolarisation (AP)
K permeability remains for several milliseconds after the spike. Vm heads towards Ek
10
New cards
Voltage-gated cation channels
K, Na and Ca ion channels each made of 4 homologous polypeptide domains which join to form a pore. E\~ach domain is made of 6 alpha helix segments.
11
New cards
Selectivity
Negatively-charged amino acids are found at the intra/extracellular openings of segments 5 and 6 to repel anions.
12
New cards
Snug-fit model (selectivity)
Selectivity filter contains for carbonyl oxygen atoms which can bind with K+ to remove the water of hydration, allowing the smaller, dehydrated K+ to pass through. Na cannot pass as it is unable to form enough bonds with the oxygen to remove all of the water, therefore too large.
13
New cards
Voltage sensitivity
Segment 4 of each domain has positively-charged amino acids that move outwards when the membrane depolarises to open the channel.
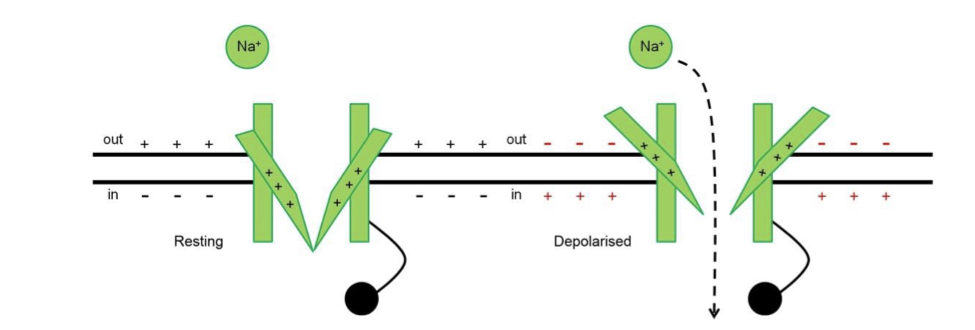
14
New cards
Hodgkin cycle
Positive feedback process where the opening of \`Na channels in a segment allows more \`Na to move into the cell causing more depolarisation and hence more Na channels open.
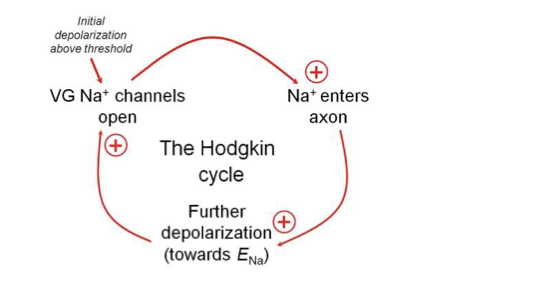
15
New cards
Inactivation
Loop of amino acids (‘ball-and-chain in K channels) swings up to block the channel on the cytoplasmic side. Cue for inactivation is the original depolarisation, but inactivation takes time, vice versa for re-activation.
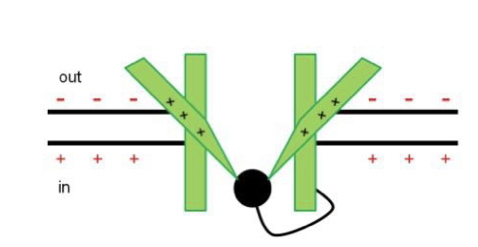
16
New cards
Differences between channels
Sodium channels have faster opening repsonse to depolarisation than potassium. Na channels also inactivate far more quickly (1-2 millisec)
17
New cards
Stochastic
Describes the behaviour of individual channels, only the probability of their state can be determined, based on membrane potential and its immediate history. However the collective behaviour of many channels is predictable.
18
New cards
Activation threshold
The initial depolarisation such that the Na entry exceeds K loss, causing the positive feedback Hodgkin cycle.
19
New cards
Absolute refractory period (ARP)
Time-period after the beginning of an AP when a second AP cannot be generated, no matter the stimulus size.
20
New cards
Relative refractory period (RRP)
Time in which a second AP can only be elicited with a stimulus of greater amplitude than normal, as a proportion of the channels are inactive, so a higher fraction of the available channels must be activated.
21
New cards
Local circuit current
Positive charge (not exact ions) spreads as driven by potential gradient, this depolarised the next segment of membrane, positive ions leave the extracellular side to complete the circuit causing Hodgkin cycle. Inactivation ensures unidirectionality.
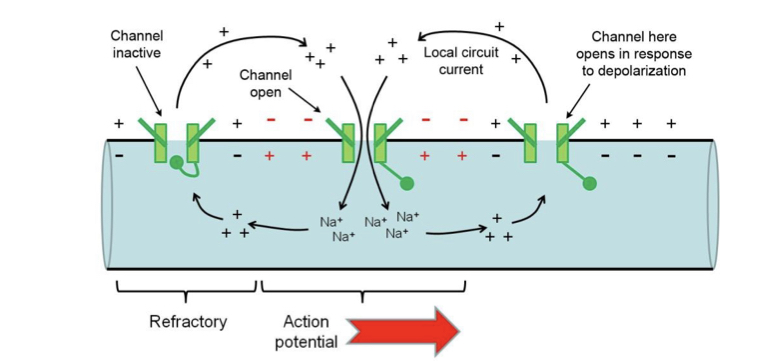
22
New cards
Electrotonic propagation
Passive process where a given depolarisation declines in size over distance. Na+ entry reinforces the signal at each successive axon segment.
23
New cards
All-or-none
If the original stimulus was above threshold, successive self-reinforcement means the AP amplitude at any distance will always be equal.
24
New cards
Electric analogue model
Each segment of an axon can be represented as 3 resistors and a capacitor.
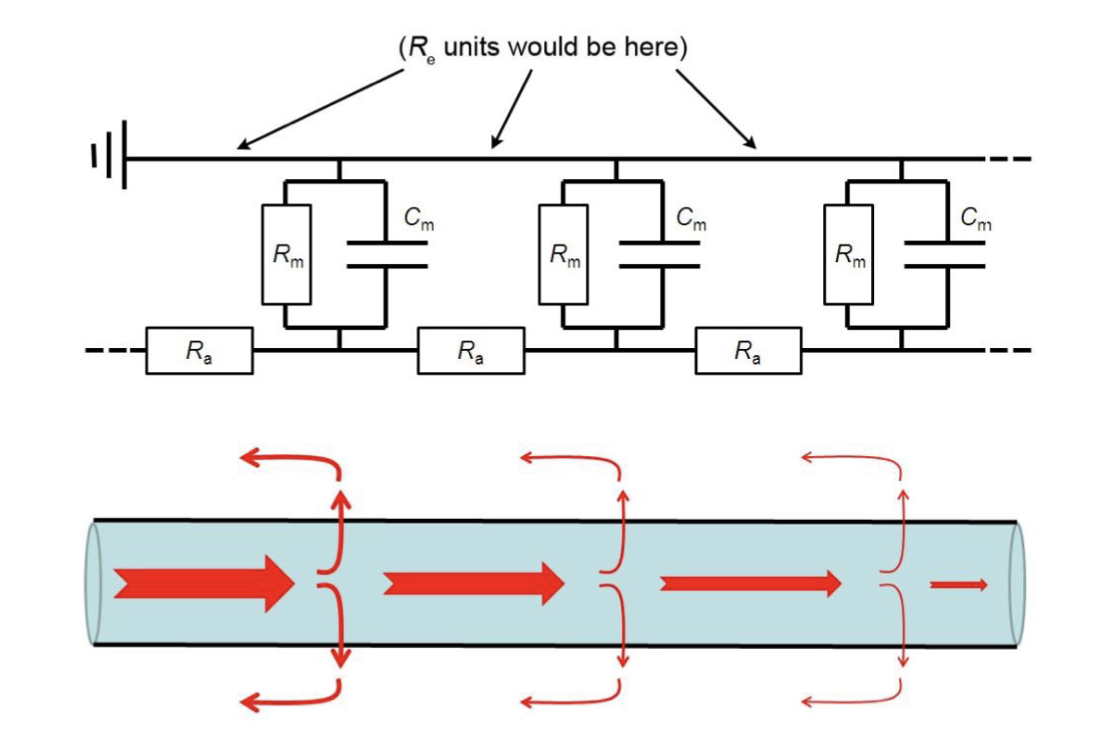
25
New cards
Re
External resistance due to extracellular fluid: negligible.
26
New cards
Ra
Axonal resistance: inversely proportional to cross-sectional area.
27
New cards
Rm
Membrane resistance per unit length of axon: inversely proportional to membrane area and density of ‘background’ ion channels.
28
New cards
Cm
Membrane capacitance: proportional to surface area.
29
New cards
Adaptations for efficient propagation
To lose less current: Less ‘leaky’ membrane and wider axon, for easier current flow.
30
New cards
Length constant (Lamda)
Distance over which the voltage change caused by an injection of current at distance x=0 decays to 37% (1/e) of its original value.
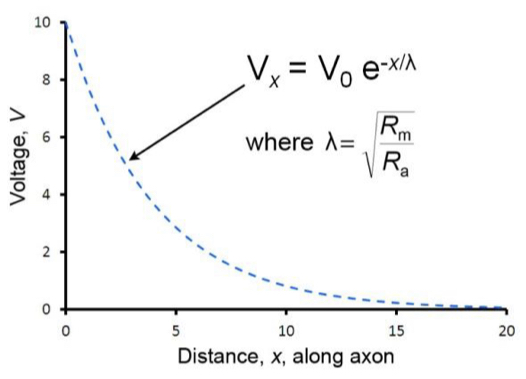
31
New cards
Time constant (Tao)
Time taken for the membrane potential to rise from baseline to 63% of its final, asymptotic value at x=lamda.
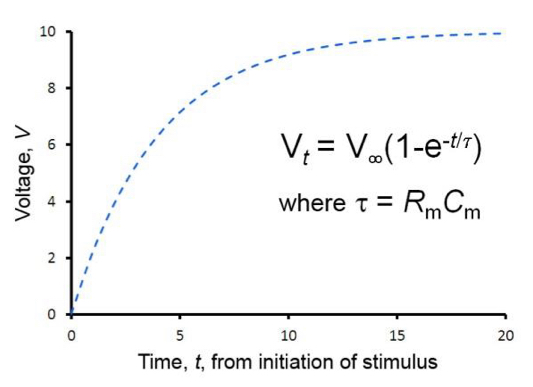
32
New cards
Myelin sheath
Layers of specialised cell membranes wrapped several hundred times around a nerve axon. From Schwann cells in the peripheral nervous system, from oligodendrocytes in the CNS.
33
New cards
Nodes of Ranvier
Non-myself aged sections between myelin internodes.
34
New cards
Internodes
Myelinated regions of a nerve axon.
35
New cards
Saltatory conduction
The ‘jumping’ of an AP by means of an extended local circuit current between Nodes of Ranvier, due to the restriction of transmembrane sodium currents to the nodes.
36
New cards
Safety factor
Myelination increases the length constant longer than it needs to be, so several nodes are excited at once during the AP.
37
New cards
Factors affecting AP conduction velocity
Behaviour/density of voltage-gated ion channels, warmer temperatures (inc), larger length constant (inc), smaller time constant (inc).
38
New cards
Metabolic advantage of myelin
Fewer Na+ cross the axons membrane therefore fewer ions need to be pumped back out by Na+/K+ ATPase.
39
New cards
Squid giant axons
Used to stimulate mantle muscles to coordinatedly contract, part of their jet-propulsion escaper response. Diameter 1mm, conduction 25m/sec.
40
New cards
Human axons
Diameter 20micrometers, speed 120m/sec.
41
New cards
Synapse
Specialised region of communication between two cells, at least one of which is excitable.
42
New cards
Electrical synapse
Ionic current can pass directly between two cells via gap junctions. Found in smooth muscle cells, cardiac muscle cells and some neurons.
43
New cards
Ionotopic transmission
Fast chemical synapses (still incur delay, 0.5-2 msec) where ion channels are opened directly.
44
New cards
Metabotropic transmission
Slower chemical synapses which involve second messengers to modulate ion channel activity.
45
New cards
Alpha motor neuron
Contains cell body in the ventral horn of the grey matter (non-myelinated) of the spinal cord. Has a large, myelinated axon as part of a somatic motor nerve to innervate skeletal muscle. At destination, axon divides to form NMJs with several msucle fibres.
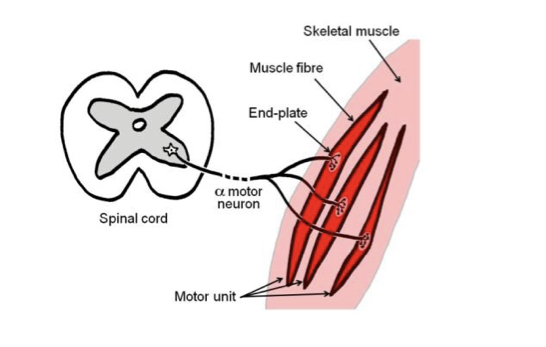
46
New cards
Motor unit
All the muscle fibres innervated by the same axon, can very from a few to a few hundred.
47
New cards
Neuromuscular junction
Consists of branching axon terminal, contained within a gutter in the muscle fibre membrane (post-synaptic membrane\`). Covered by the cytoplasm (but not membrane) of the last Schwann cell (supports physically and chemically). Also referred to as motor end plates.
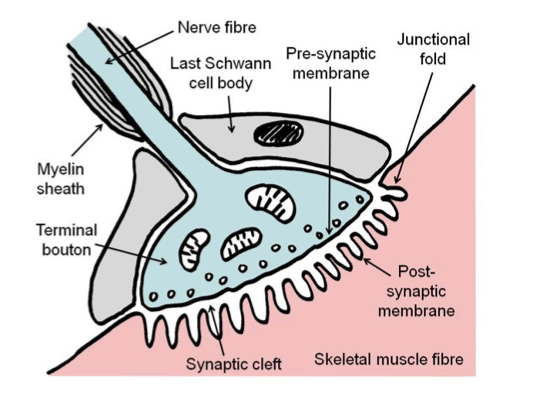
48
New cards
Terminal bouton
Multiple swellings within the end-plate of the axon, release neurotransmitter acetylcholine.
49
New cards
50 nm
Width of a synaptic cleft.
50
New cards
Synaptotagmin
Calcium sensor found on the membrane of secretory vesicles. Promotes interaction between target-membrane SNARE (t-SNARE) and vesicle-membrane SNARE (v-SNARE). Leading to exocytosis.
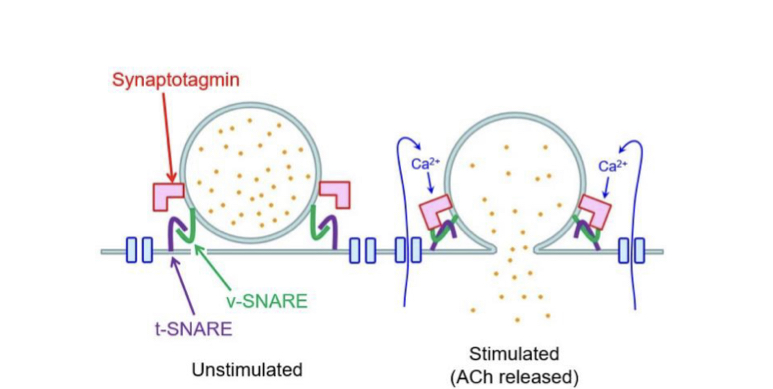
51
New cards
Action at terminal bouton
Depolarisation causes voltage-gated calcium ion channels to open, these are located close to the active zone where synaptic vesicles are held.
52
New cards
Nicotinic acetylcholine receptors (NAChR)
Ligand gated ion channels with a high density on the crests of junctional folds. Pentameric structure in adult mammals.
53
New cards
Action of NAChR
One ACh must bind to each of the 2 alpha subunits causing conformational change and channel opens. Allows Na+ and K+ to pass (opposite directions) causing depolarisation 30 times faster than Na+ channel.
54
New cards
Botulinum toxin (Botox)
Neurotoxin that prevents presynaptic release of acetylcholine
55
New cards
End-plate potential (EPP)
Depolarisation of the post-synaptic membrane at about 20-40 mV, and propagates electrotonically and hence locally to nearby Na channels.
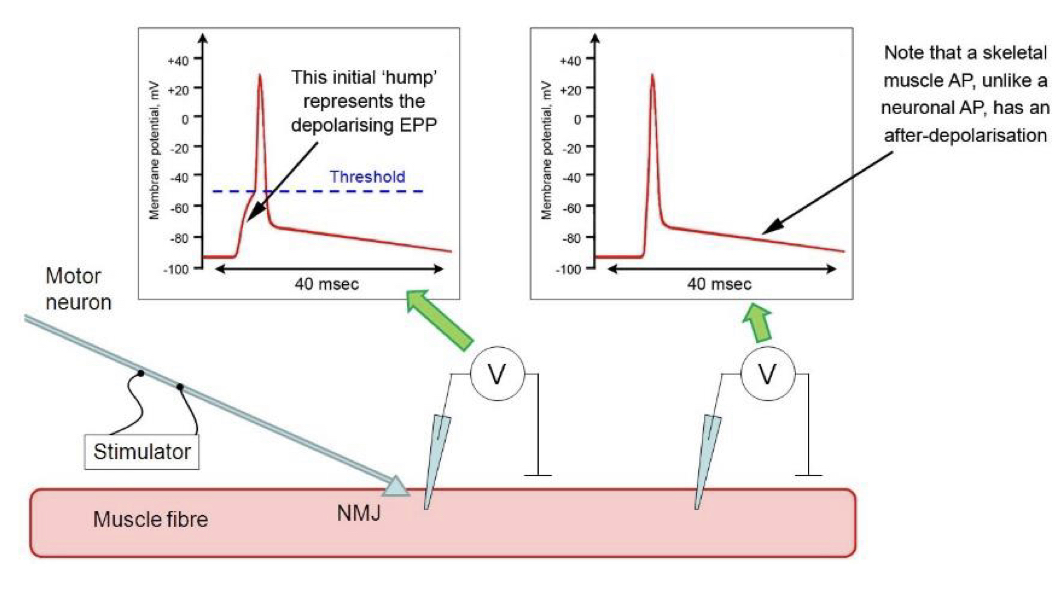
56
New cards
Tubocurarine
Drug used to reduce the size of an EPP below threshold so that an action potential is not generated and the hump of the EPP can be measured.
57
New cards
Miniature end-plate potentials (mEPPs)
Spontaneous release of singular vesicles fusing with the pre-synaptic membrane (0.4mV potentials). Stochastic event following a Poisson distribution.
58
New cards
Acetylcholinesterase
Enzyme that breaks down the released ACh to choline and acetate.
59
New cards
Choline recycling
Choline is actively transported across the pre-synaptic membrane and recycled into ACh using acetate from acetyl coenzyme A
60
New cards
Terminating response
ACh break down by acetylcholinesterase, new vehicles made by endocytosis, transporters fill them with newly synthesised ACh, Ca2+ is actively pumped out of the terminal of the nerve.
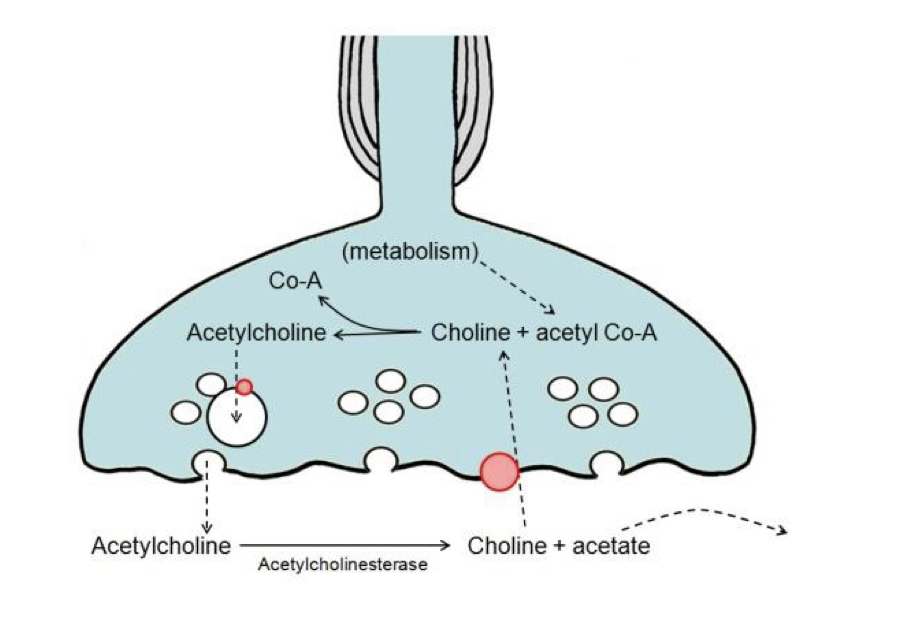
61
New cards
Central neurons
Neurons that work by integrating information from multiple synaptic inputs into their branching dendrites.
62
New cards
Excitatory post-synaptic potentials (EPSPs)
Small depolarisations of the dendrites, increase the probability of reaching threshold and AP firing. Temporal and spatial summation occur which can be large enough to bring the membrane to threshold.
63
New cards
Inhibitory post-synaptic potentials (IPSPs)
Decrease probability of cell reaching threshold, sometimes hyper polarising. Use GABA (brain) or glycine (spine) to increase Cl- permeability
64
New cards
EPSPs and IPSPs
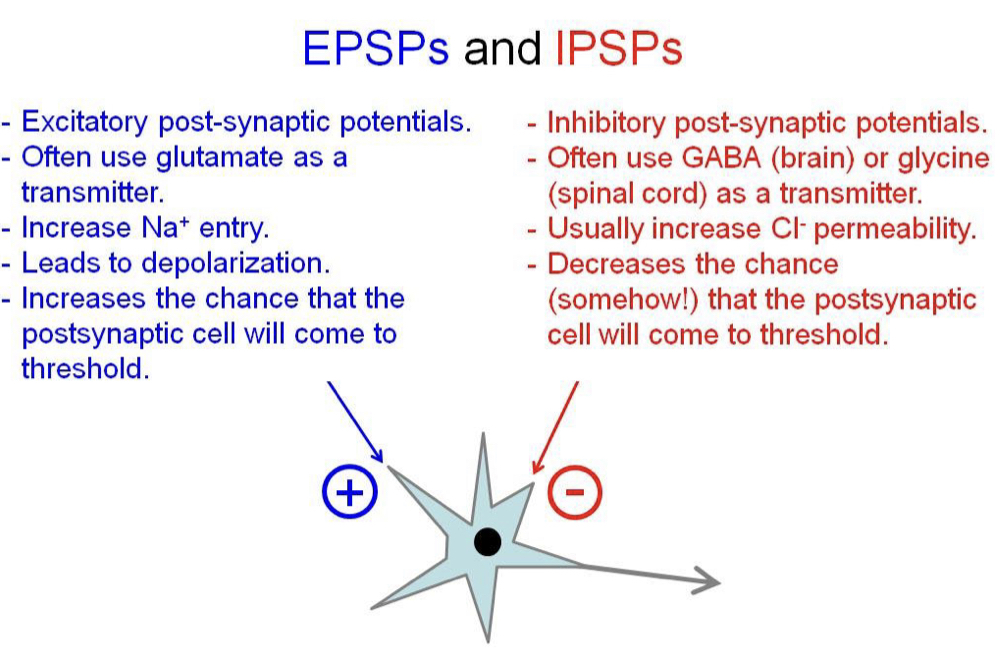
65
New cards
Axon initial segment
Site of AP initiation on a central neuron, located past the axon hillcock. Has a high density of voltage-gated Na channels.
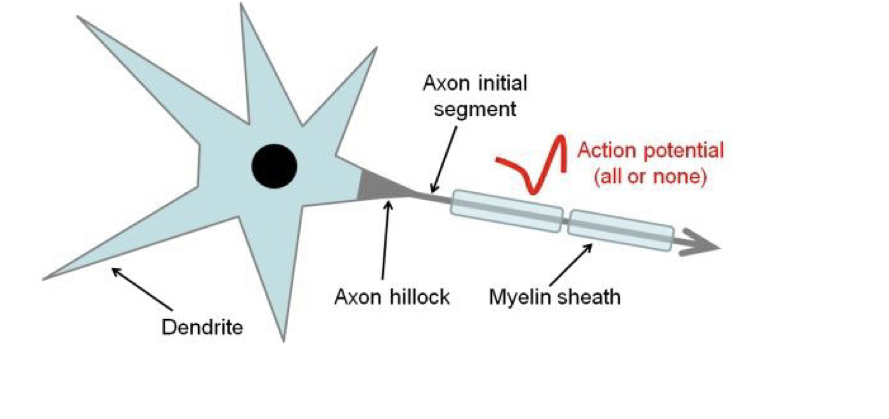
66
New cards
Myogenic
Electrical signals generated by muscle rather than nerve impulse. Like APs generated by cardiac muscle cells.
67
New cards
Sinoatrial node (SAN)
Pacemaker of the heart: group of modified, non-contractile myocytes within the wall of the right atrium. Connected to normal cardiac myocytes by gap junctions. (SAN ensures coordination)
68
New cards
Pacemaker potential
SAN cells do not have a resting potential, as their membrane slowly and spontaneously depolarises due to influx of Na (funny current) followed by Ca (through L-type voltage-gated Ca channels). This can initiate an AP.
69
New cards
Repolarisation of SAN cells
Occurs when calcium channels inactivate and K leaves via voltage-gated ‘delayed rectifier’ channels
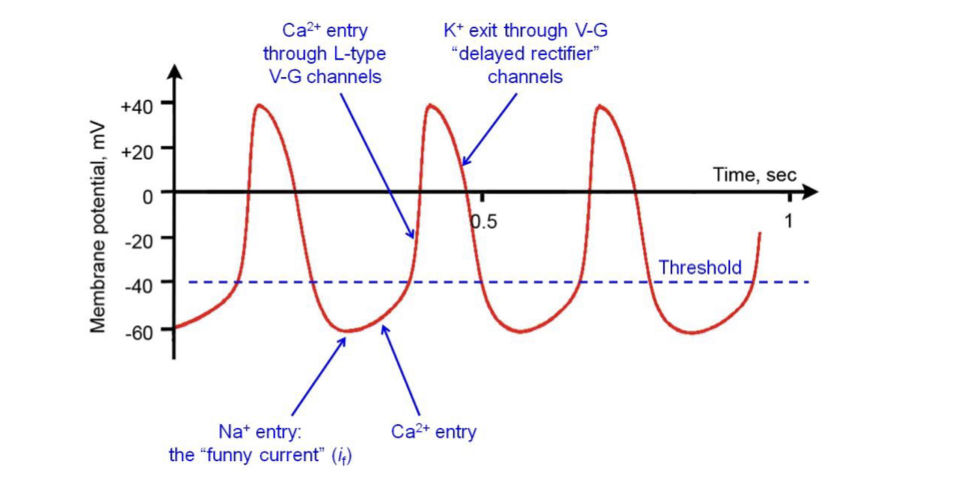
70
New cards
Autonomic nervous system and SAN
Controls the slope of the pacemaker potential and heart rate.
71
New cards
Autonomic nervous system (ANS)
Innervates smooth muscle, cardiac muscle and various secretory glands. 3 divisions: sympathetic, parasympathetic and enteric, with the first two both being efferent.
72
New cards
Alpha motor neurons
Nerve fibres that supply skeletal muscle, cell bodies reside in the ventral horn of the grey matter of the spinal cord. (Somatic nervous system)
73
New cards
Ventilation
Involved in the homeostatic control of blood gas concentrations and pH, requires the use of the skeletal diaphragm and chest-wall muscles.
74
New cards
Shivering
Homeostatic control of body temperature mediated by skeletal muscles.
75
New cards
Preganglionic neuron
Myelinated neurons with a cell body in the CNS. Sends axon to an **autonomic ganglion**, where there is a cholinergenic synapse. In both sympathetic and parasympathetic.
76
New cards
Postganglionic neurons
Cell bodies in the ganglion and unmyelinated axons innervate the muscle/gland in question.
77
New cards
Difference between parasympathetic and sympathetic nerve fibres
Sympathetic= short preganglionic and long postganglionic. Releases adrenaline or noradrenaline
Parasympathetic= long preganglionic and short post ganglionic. Releases ACh at terminal to a muscarinic receptor.
Parasympathetic= long preganglionic and short post ganglionic. Releases ACh at terminal to a muscarinic receptor.
78
New cards
Terminal varicosites
Used in place of a NMJ, acts as a ‘neuroeffector junction’ with the target tissue.
79
New cards
Sympathetic nerve fibres
Cell bodies in the intermediolateral column of the spinal cord grey matter. Axons are sent out via the ventral root.
80
New cards
Noradrenaline
Released as a neurotransmitter by postganglionic neurons, also released by the adrenal medulla but does not have a physiologically significant effect and hence is not considered a hormone.
81
New cards
Adrenaline
Some preganglionic sympathetic fibres synapse onto chromaffin cells in the adrenal medulla, ACh causes these cells to release adrenaline into the blood (endocrine arm of the SNS)
82
New cards
Chromaffin cells
Specialised ganglion cells
83
New cards
G-protein coupled adrenergic receptors
Receptors affected by adrenaline and noradrenaline.
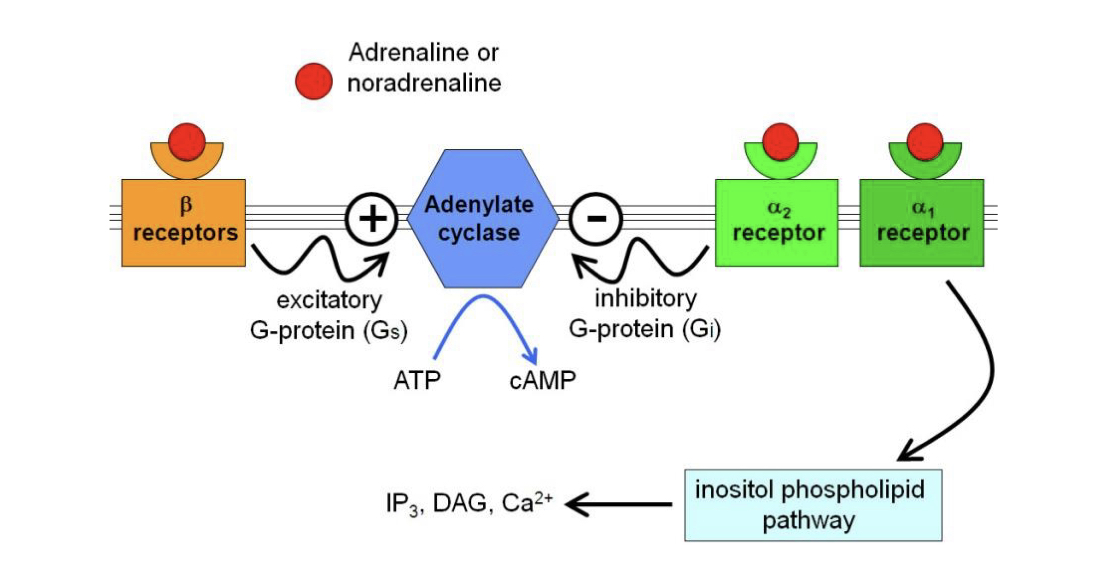
84
New cards
Alpha-2 and beta-2
Receptors for which adrenaline is more potent
85
New cards
Alpha-1 and beta-1
Receptors for which noradrenaline is more potent
86
New cards
Caltecholamines
Grouped name for adrenaline and noradrenaline
87
New cards
Beta receptors
Coupled to excitatory G-proteins which increase cAMP as a second messenger inside the cell via adenylate cyclase
88
New cards
Cyclic AMP
The second messenger in G-protein coupled receptors
89
New cards
Alpha 1 receptor
Works via Gq to activate the inositol phospholipid pathway
90
New cards
Inositol phospholipid pathway
Key pathway within cell signalling
91
New cards
Alpha 2 receptor
Receptor coupled to an inhibitory G-protein inside the cell, which impacts adenylate cyclate to produce less cAMP
92
New cards
Factors affecting the effects of adrenaline
1. Which cells express adrenergic receptors
2. How many are expressed
3. What kind they are
93
New cards
Preganglionic parasympathetic fibres
Myelinated fibres that emerge from the brain and are carried within cranial nerves to their target organs.
94
New cards
Vagus nerve
Nerve which supplies parasympathetic innervation to most of the thoracic and abdominal organs.
95
New cards
Synapse of preG parasympathetic fibres
Synapse either in a ganglion close to the target organ or within the wall of the organ itself. (ACh acts on NAChR at the synapse)
96
New cards
Muscarinic cholinergenic receptors
Receptors for which the postganglionic fibres of the parasympathetic nervous system release ACh (or sometimes vasoactive intestinal peptide or nitrogen oxide)
97
New cards
Atropine
Drug which blocks muscarinic receptors.
98
New cards
Enteric nervous system
Responsible for gastrointestinal innervation, for example the parasympathetic postganglionic fibres going to the gut belong to it.
99
New cards
Heart at rest
Receives tonic parasympathetic stimulation from the vagus, but little if any sympathetic stimulation.
100
New cards
Effect of catechomines on the heart
Beta-1-adrenoreceptors on SAN cells respond to adrenaline and noradrenaline by activating an excitatory G-protein, leading to an increase in intracellular cAMP. This results in Na and Ca channels opening, speeding up the rate of depolarisation and therefore heart rate.