Exam 1
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
MI
Acute onset of myocardial ischemia that results in myocardial cell death
Infarction – occlusion in vessel that causes tissue death
MI Patho
Plaque rupture ➡ platelet activation ➡ thrombus formation ➡ complete vessel occlusion
Necrosis of myocardial cells supplied by the occluded vessel
Use of Aspirin
Prevents the platelet aggregation
Makes it harder for platelets to stick
Necrosis is irreversible
Results in non-functional area of myocardium
Can lead to HF
Infarcted area can longer act as a pump or conduct electricity
Increases risk of dysrhythmias
If the thrombus completely occludes the vessel, this will likely cause a STEMI.
If the clot partially occludes the vessel, then this is manifested as unstable angina/NSTEMI
coronary arteries
RCA goes to front and wraps around the back
LCA divides into two branches
LAD goes down the front anterior wall
Circumflex feeds the lateral wall and swings to the posterior wall
Widowmaker = occlusion in proximal LCA
CAD patho
Prolonged ischemia >20 minutes ->cellular death begins.
Ischemic tissue
May not have normal contractility
Has an increased risk of dysrhythmia formation
Infarction develops over minutes to hours
Death of cells- enzymes are released
Enzymes are:
Troponin
Creatine Phosphokinase (CPK)
Myoglobin
Lactic Dehydrogenase (LDH)
type of mi based on ekg
ST elevation MI
STEMI
Transmural or full thickness
Non-ST elevation MI
NSTEMI
Subendocardial – partial thickness
acute MI symptoms
Chest pain
Sudden, severe, crushing, unrelieved by rest or nitroglycerin
Often radiates to one or both arms, jaws, neck and back.
No correlation between severity of pain and severity of infarction
New murmur, S3 or S4 heart sounds
Infarctions can involve the papillary muscles
S3 -> HF
JVD and SOB
Crackles
S4 -> HTN
If heart failure is present – JVD, SOB
Dysrhythmias
BP ↑ or ↓
Pain and increased sympathetic reaction
Large amount of damage can increase BP
EKG changes
MI clinical presentation
Shortness of Breath/pulmonary edema
Diaphoresis
Palpitations
Nausea/vomiting
Can be projectile
Anxiety, feeling of pending doom
Skin cool, clammy
Cardiac arrest
Shock
Dysrhythmias
Low grade fever – later sign
24-48 hours later
Cardiac enzyme elevation
EKG changes
Vital Signs
brief exam
ABC’s, vital signs, general observation
Neuro
Stroke symptoms, restlessness, lightheaded, anxiety
Cardiac
Chest pain, EKG changes, JVD, murmurs, S3, S4, pulses
Pulmonary
Crackles, resp distress, tachypnea, pulmonary edema
Gastrointestinal
Nausea/vomiting
Genitourinary
Urine output
Skin
Cool, clammy diaphoresis, pale
Psychosocial
Feeling of impending doom, fear, denial
causes of CP not related to ACS
Aortic Dissection
Pulmonary Embolism
Esophageal Rupture
Pneumothorax
atypical presentations
Women
Lethargy
Weakness
Nausea
Elderly
May not feel the CP
Diabetics
May not feel the CP
Transplanted Heart
Will never have anginal CP
Get heart cath every year
EKG diagnosis
Ischemia
ST segment depression
T wave inversion
Nonspecific sign
Antidysrhythmic drugs can cause this
Injury
ST segment elevation
Injury = beginning of the MI
Tissue is still viable
Infarction
Pathologic Q waves develop permanently with STEMI
Irreversible damage
cardiac enzymes
Serum Enzymes
Enzymes are released as cellular necrosis occurs.
Troponin
Very Specific for Cardiac Cellular Death
Rises 3-6 hours after injury
Peaks in 12-18 hours
Stay elevated for 1-2 weeks
Other enzymes that rise:
Creatine Phosphokinase CPK
Lactic dehydrogenase LDH
Myoglobin
STEMI treatment
Rapid transport to the hospital – activate EMS
12 Lead EKG – to be read within 10 min
Obtain blood for cardiac biomarker – troponin
Routine medical interventions (MONA)
MONA is not definitive treatment but is for initiating treatment
Morphine, Oxygen, Nitroglycerin, Aspirin
Oxygen
If O2 is < 94
Morphine
Pain and vasodilates
Aspirin – chewable 162mg to 325 mg
antiplatelet
Nitroglycerin
Check BP
Sublingual 0.4 Q5 after checking BP if pain is unrelieved
only give If SBP is > 100
Slight burning is normal
other STEMI tx
Beta blockers
IV
Decrease workload on the heart
Avoid v-fib
To slow HR
The slower the hr the more time in diastole
Diastole is when the heart is being perfused
Anticoagulation
Clopidogrel, heparin, glycoprotein IIb/IIIa agents (Eptifibatide/Integrilin)
reperfusion therapy
Reperfusion – for eligible patients with symptoms within the past 12 hours
PCI capable facility– door to balloon time 90 minutes
Percutaneous coronary intervention in the cath lab
Non-PCI capable facility – transfer to PCI capable facility if < 120 minutes to balloon time
If PCI cannot be done in < 120 minutes, administer fibrinolytics
Dissolve the clot
If fibrinolytics are given, they should be administered within 30 minutes of arrival to hospital. (Door to needle time)
MI nursing considerations
IV access
Administer meds
Monitoring vital signs/hemodynamic stability
Enough blood to perfuse organs (brain, kidney, etc.)
Continuous cardiac monitoring
Labs
Troponin
Electrolytes, BUN, Creat, CBC with platelet ct, INR, Magnesium, Glucose, Serum lipids, aPTT
Patient support and education
PCI stent
Invasive Procedures
Coronary stent placement
Done during the cardiac catherization
Permanent
Give Plavix/clopidogrel to avoid clotting in the stent
PCI post care
Monitor for dysrhythmias, chest pain, neurologic changes
Bedrest for at least 4 hours
HOB no higher than 30 degrees
Frequent VS checks every 15 min x’s 4, every 30 min x’s 4 and every hour x’s 4
Assess distal pulses with each VS check
On the side of the pci
Assess for bleeding or hematoma with each VS check
May not see the tachycardia bc of the meds
Monitor I/O
Increase fluid intake or IV fluids to prevent contrast induced nephropathy
Acetylcysteine – Mucomyst
PCI complications
Hematoma
Vascular complications
Embolism
Hypersensitivity to contrast dye
Steroids, Benadryl, Pepcid to premedicate
Dysrhythmias
Bleeding
Restenosis
CP
Stroke
Contrast induced nephropathy
fibrinolytics
Activate the body’s own fibrinolytic system to lyse coronary clots
Diagnosis of STEMI must be confirmed.
Not for an NSTEMI
Done if PCI cannot be done within 120 minutes
Damaged tissues, blood vessels or organs stimulate platelet aggregation
A platelet plug forms over damaged vessel
Clotting cascade is initiated
Vasoconstriction occurs
Fibrin surrounds platelets to form a clot
Fibrinolytics activate the body’s fibrinolytic system to lyse a blood clot
Dissolution of fibrin clot is done by plasmin.
Plasmin is circulating in the body in the inactive form of plasminogen.
Plasminogen is activated by tissue plasminogen activator (TPA) which converts the plasminogen into plasmin which digests the clot.
Early administration limits infarct size, reduces myocardial damage and improves outcomes
absolute contraindications for fibronolytics
Prior intracranial hemorrhage/hemorrhagic stroke
Known cerebral vascular lesion or neoplasm
Ischemic stroke with in the last 3 months
Suspected aortic dissection
Active bleeding
Recent major surgery or trauma
Severe uncontrolled hypertension (unresponsive to emergency therapy)
Known bleeding disorders
Pregnancy
intracranial hemorrhage risk factors
Worst complication for fibrinolytics
Age>65 years
Weight > 70kg
Female gender
HTN on admission
evidence of reperfusion
Reperfusion dysrhythmias (PVCs, accelerated idioventricular rhythm)
Generally, not life threatening and not typically treated
Abrupt cessation of chest pain
Though may not be a reliable indicator especially when morphine us used.
Pain is subjective
Rapid return of ST segment to baseline
Very good indicator of reperfusion
Best predictor of reperfusion is the cessation of chest pain coupled with ST segment return to baseline
post TPA infusion care
Frequent assessment of VS
Continuous EKG monitoring
Observe for evidence of bleeding - ↓BP & ↑HR
Assess body fluids for evidence of bleeding
Flank pain/back pain – may indicate retroperitoneal bleeding
Assess LOC – intracranial bleed
Can be subtle like anxiety and restlessness
Assess puncture sites for bleeding
Anticipate the need to apply additional pressure at puncture sites
Evaluate response to therapy
Report manifestations of re-occlusion
Minimal handling of the patient
Bedrest for 6 hours
Avoid injections
Best to insert 2 IVs prior to administration
Prophylactic H-2 blockers
Anticoagulation may be recommended after fibrin therapy to improve vessel patency and prevent re-occlusion.
IV Heparin - monitor aPTT
Enoxaparin
indications for PCI capable facility post TPA
Development of cardiogenic shock
Urgent transfer for failed reperfusion
In stable patients between 3 and 24 hours after successful fibrinolysis
complications for acute MI
Cardiogenic shock
Heart Failure
Dysrhythmias
Ventricular
Slow – Bradycardia, AV heart block
Pericarditis
Papillary muscle rupture
Wall rupture
Septum
Ventricular free wall
LV aneurysm
Bleeding
AKI
Anoxic brain injury if there was a cardiac arrest
HF/pulm edema
Infarcted tissue does not pump
Acutely may require vasopressor support and intubation
Treat with medications
ACE Inhibitors
Beta Blockers
Diuretics
Aldosterone Antagonists
MI nursing care
Cardiac Assessment
Vital signs
Evaluation and relief of chest pain
Assessing for heart failure
Monitoring for dysrhythmias
Evaluation of adequate perfusion
Level of consciousness
Adequate urine output
Most important
Gastrointestinal symptoms
Skin temperature
Capillary refill
Nursing care for chest pain
Assess and document CP
Vital signs including heart rhythm
Assess skin temp
12L EKG
Decrease physical activity
Priority if they do not need the oxygen
Oxygen, NTG, Morphine
Provide a restful environment
Small meals
Assist with ADLs
Avoid straining – stool softeners
Help patient to relax
Teach patients to recognize symptoms
MI discharge meds
Statin therapy
Lowers cholesterol
Provide plaque stabilization
Stabilize their plaque
LFT, and Rhabdo
Angiotensin converting enzyme inhibitors
If MI is anterior wall
HF and an EF < 40%
Aldosterone antagonists
EF < 40%
Symptoms of HF
Diabetes
spironolactone
Dual Antiplatelet Therapy (DAPT)
Aspirin
Clopidogrel (Plavix)
MI discharge
Call 911 for CP/SOB and associated symptoms
Seek immediate care for
Unusual fatigue
Rapid pulse
Bleeding
Urine, stool, nose,etc.
Low urine output
New or increased swelling in feet or ankle
Medications
Take as prescribed
Notify provider for side effects of medications
Education on medications
Indications
Side effects
Lifestyle modifications
Heart healthy diet
Smoking cessation
Exercise – cardiac rehab
Maintain ideal body weight
BMI 18.5-24.9
Manage stress
annual flu shot
Keep follow up appointments
HF disparities
African Americans have higher rates for HF
Ages 65-69 – 20/1000 have HF
Age >85 80/1000 have HF
LGBTQ adults have increased rates of smoking than heterosexual peers
Transgender adults have lower levels of physical activity than heterosexual peers
Lesbian and bisexual women have higher rates of obesity than heterosexual women
HF
a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood.
Characterized by
Fluid volume overload
Inadequate tissue perfusion
Cardinal Manifestations are:
Dyspnea and fatigue
Exercise intolerance
Fluid retention
Peripheral edema
ejection fraction
percentage of blood that is ejected from the ventricles during systole
EF=SV/EDV
55-70% - Normal limits
40-55% - Below normal
<35% - Increased risk of life-threatening arrhythmias
Cardiac Output = HR x SV
preload and afterload
Preload - the degree of stretch of the ventricular cardiac muscle fibers at the end of diastole
The volume of blood in the ventricle at the end of diastole determines preload
Diuresis decreases preload
Fluid bolus increases preload
Afterload – the resistance to ejection of blood from the ventricle
HTN and stenosis increase afterload
In heart failure both preload and afterload can be increased
HF patho
HF begins after an event produces a decline in the heart’s pumping capacity.
Can have an abrupt onset or a gradual insidious onset
Contractility decreases resulting in an increased end diastolic volume
Causes increased stretching and dilation of the ventricular muscle tissue
Compensatory mechanisms are activated.
The body’s attempt to maintain cardiac output
Treatment for HF is aimed at blocking these compensatory mechanisms
Compensatory mechanisms
Short term can restore cardiac function to a normal or near-normal range.
Over time, compensatory mechanisms can lead to left ventricular remodeling and cardiac decompensation
neurohormonal compensatory mechanisms
Baroreceptors
Decreased flow causes stimulation of the SNS
Sympathetic nervous system (SNS)
Initially compensates by increasing heart rate and peripheral vascular resistance.
Over time, catecholamines cause toxic effects on the myocardium
Direct toxicity to the myocardium
Myocardial remodeling & facilitation of dysrhythmias
Renin angiotensin aldosterone system
renal perfusion causes the kidneys to release renin.
Renin acts on angiotensinogen to form angiotensin I.
In the presence of angiotensin converting enzyme, angiotensin I is converted in the lungs into angiotensin II.
Angiotensin II causes vasoconstriction, sodium and water retention and pro-fibrotic and pro-inflammatory effects that contribute to cardiac remodeling. Also stimulates the release of aldosterone
Aldosterone
Angiotensin II stimulates the release of aldosterone from the adrenal cortex which causes sodium and water retention.
Aldosterone is associated with cardiac fibrosis and ventricular remodeling
causes of HF
Hypertension
Diabetes Mellitus
Metabolic Syndrome
Atherosclerotic Disease – CAD, MI – CAD is found in the majority of those with heart failure
Cardiomyopathy
Dilated, Ischemic, familial, alcohol-induced, cocaine, toxic, chemotherapy, tachycardia-induced, peripartum, iron overload, Stress (Takotsubo)
Valvular Disease
Thyroid Disease
Myocarditis
HIV
Rheumatologic/Connective Tissue Disorders
Amloidosis/Scarcoidosis
natriuretic peptides
Atrial natriuretic peptide
B-Type natriuretic peptide (BNP)
Normal < 100
Naturesis = sodium excretion
Beneficial but not sufficient to overcome other pathways
Hormones that are secreted due to myocardial wall stretching.
They have beneficial effects of natriuresis and vasodilation
systolic failure
(HFrEF)
Inability of the heart to generate adequate cardiac output to perfuse vital tissues.
Decreased contractility causes a decrease in stroke volume and an increase in left ventricular end-diastolic volume.
Over time the increased volume causes dilation of the heart
diastolic failure
(HFpEF)
The left ventricle loses the ability to relax normally and can’t properly fill with blood during diastole
There are no approved pharmacologic or device therapies for the management of patients with HF with preserved EF
Dyspnea should be treated with sodium restriction and gentle use of diuretics
R vs L sided HF
Generally symptoms are seen together
Right
LHF common cause of RHF
Jugular venous distension
Peripheral edema
Ascites
Hepatomegaly
Jaundice
Weight gain
Left
Problem with forward flow and blood backing up into lungs
Pulmonary symptoms
Pulmonary edema
Pink frothy sputum
Fatigue
Exercise intolerance
Cyanosis
Orthopnea
Paroxysmal nocturnal dyspnea (PND)
Weak pulses
Decreased perfusion to vital organs
Decreased urine output (oliguria or anuria)
LOC changes
Cool clammy
HF classes
NYHA
I - Asymptomatic
II - Minor Symptoms, onset with modest exertion
III - Moderate symptoms, onset with minor exertion
IV - Symptoms at rest
ACC/AHA
A - at risk of HF but no structural disease
B - Structural HF with no symptoms
C - Structural HF with current or prior symptoms
D - Symptoms at rest
HF manifestations
Neurological
Fatigue
Depression
Cardiovascular
Laterally displaced PMI
S3
Early diastolic heart sound
Overfilling of ventricles creates a new sound
Jugular venous distention
Diminished pulse pressure
Heart cannot create systolic pressure
Peripheral edema
Dysrhythmias
Vital Signs
Tachycardia
Generally hypotensive
Respiratory
Orthopnea
Paroxysmal nocturnal dyspnea
Acute pulmonary edema
Pulmonary crackles, wheezing
Cough
Pleural effusions
Gastrointestinal
Hepatomegaly
Ascites
Jaundice
Cardiac cachexia
Weight gain
Renal
Decreased urine output
Renal dysfunction
HF diagnosis
History and Physical Exam
Routine laboratory testing
Electrolytes, BUN, creat, CBC, hepatic enzymes, BNP, Thyroid function testing
12 Lead EKG
Ischemia, ekg changes, past MI
CXR
Cardiac enlargement
Pulmonary arteries distended
Assessment of cardiac structure and function – transthoracic echocardiogram (TTE)
Ischemic work up to determine reversible causes
Want to ensure stents are not placed in already dead tissue
HFrEF treatment
Diuretics
ACEIs
ARBs
Beta Adrenergic Receptor Blockers
Aldosterone Antagonists
Nitrates and Hydralazine
ARNI
SGLT2i
Digoxin
Inotropes
Anticoagulation Antiplatelet Therapy
diuretics
The only pharmacologic agents that adequately control fluid retention
Loop diuretics - furosemide, bumetanide, torsemide
Hypokalemia -> dysrhythmias
Hypotension if already have a borderline BP
Thiazide diuretics – metolazone
Increases potency of loop diuretic
Potassium sparing diuretics – spironolactone, eplerenone
Aldosterone blockers
MOA
inhibiting the reabsorption of excretion of water and electrolytes, primarily sodium
Preload and afterload
preload decreases with lower fluid volume
afterload decreases with decrease in BP
ACE inhibitors
Decreases afterload
MOA
Prevent the conversion from angiotensin I into angiotensin II.
Check BP first
Drs like lisinopril because it's a once a day dose = more adherence
Stabilize LV remodeling
Improve symptoms
Reduce hospitalizations
Prolongs life
Adverse Effects – hypotension, cough, angioedema, renal dysfunction, hyperkalemia
ARBs
decrease afterload
MOA
prevent angiotensin II from binding
Used when patients are intolerant to ACE inhibitors.
Blocks the effects of angiotensin II on the angiotensin receptor.
Stabilize LV remodeling
Improves patient symptoms
Prevents hospitalization
Prolongs life
Not to be used with ACEI!!!
Adverse Effects – hypotension, azotemia, hyperkalemia
Beta Adrenergic Receptor Blockers (beta blockers)
Always check HR and BP
reduce afterload
MOA
block epi and norepi → decreases HR
decrease contractility
slow conduction through AV node
When given in combination with ACE inhibitors:
Reverse LV remodeling
Improve patient symptoms
Prevent hospitalization
Prolong life
Adverse Effects – Hypotension, bradycardia, heart blocks. Not for use in pts with active bronchospasm. Can mask hypoglycemia
Most are cardio selective -> only affect b1 (heart) but can affect b2 (lungs)
aldosterone antagonists
MOA
reduce aldosterone which plays an important role in the pathophysiology of HF.
Diuretic effect
Prevents hospitalizations
Reduces mortality
Adverse effects – Hyperkalemia, gynecomastia, menstrual irregularities in spironolactone
nitrates and hydralazine
Used in African American patients with systolic dysfunction in addition to standard therapy.
Used for patients who are taking ACEI and BBL for symptomatic HF and who have persistent symptoms.
For HF patients with are unable to tolerate ACEI and ARBs
nitrates
significantly decrease preload by dilating venous vessels → less volume in heart
hydralazine
significantly decrease afterlaod by vasodilation
ARNI
Sacubitril/valsartan (Entresto)
Used in place of an ACEI or ARB
Works by impairing neprilysin which degrades natriuretic peptides
Adverse effects – angioedema, hypotension, impaired renal function
reduces both preload and afterload
SGLT2i
Sodium-glucose cotransporter-2 inhibitors (SGLT2i)
Dapagliflozin (Farxiga)
Reduces CV death and heart failure in those with and without heart failure and diabetes
MOA
increase glucose secretion by inhibiting SGLT2 → diuresis, decrease in BP, decrease in fluid volume
reduces preload with diuresis
digoxin
Indicated for use in patients with HF and atrial fibrillation
MOA
inhibits the Na+/K+ ATPase pump in the cardiac myocytes → increase in intracellular sodium and increased calcium inside the cell
Positive inotrope but drops HR
Considered for patients who have signs or symptoms of HF while receiving standard therapy.
Use of digoxin does not improve mortality
Toxicity can lead to dysrhythmias and hypokalemia increases toxicity
minimal effects of preload and afterload
inotropes
Affect contraction
Positive -> increase contraction
Negative -> decrease contraction
Dobutamine
Stimulates beta 1 and 2 receptors
Increases C.O.
reduces afterload
Given as a continuous IV infusion 2-10 mcg/kg/min.
Milrinone
Phosphodiesterase III inhibitor
Increases Ca in the cardiac cells and vasodilates
Decreases afterload
Check BP frequently – typically Q2 vitals
Increases cyclic AMP
Increases C.O. and has vasodilator effects.
Given as a continuous IV infusion 0.25 – 0.75mcg/kg/min
anticoagulant therapy
Patients with A fib or other risk factors for stroke should be anticoagulated
Aspirin – may be used in ischemic disease
HF nursing care
Daily Weights
2-3 pounds in a day or 4-5lbs in a week patient need to call a provider
I and O’s
Monitoring symptoms,
electrolytes/renal fx
Respiratory sx
Especially important to know if SOB is infection, HF,
If cough is infection or from ACEI
Monitoring medication effectiveness and side effects
Assessing support systems
Managing edema – thromboembolic hose, elevation of legs
Education
Diet
Medications
Signs and symptoms
Weight reduction
Exercise
Sexuality
Depression
Managing other comorbidities
Smoking cessation
device therapy
Internal Cardiac Defibrillators
Patients with an EF <35% are at risk for dysrhythmias and sudden cardiac death
Only for sudden arrest
Biventricular Pacing (Cardiac Resynchronizing Therapy)
Benefits patients with left ventricular systolic dysfunction and a wide QRS complexes who are in NSR
Improves CO
Decreases mortality
Decreases hospitalization
Reverses LV remodeling
HF preventative behaviors
Routine hand washing
Dental health
Immunizations
dysrhythmias
Definition
Disorders of the formation of electrical impulses in the heart
Disorders of conduction of electrical impulses in the heart
Both of the above
Consequences – may have hemodynamic instability
cardiac conduction system
SA node is automatic pacemaker
Spontaneously generates impulses because of permeability to sodium ions (60-100)
AV node
Slow conduction
Keeps SA node in check
Allows diastole
Back up pacemaker (40-60)
Purkinje fibers
Backup backup pacemaker (20-40)
dysrhythmia principles
The EKG provides information only about electrical events in the heart
It provides no information about contractile events
Always assess the patient for any change in cardiac rhythm
Tachycardias
Can be detrimental because
Increase myocardial oxygen demand
Decrease ventricular filling time
Decrease coronary artery perfusion time
Bradycardias
Can be detrimental because
Decrease cardiac output
Can cause syncope, chest pain
Remember CO=HR x SV
Refractory periods
Absolute refractory period
QRS complex
The heart is being depolarized
Cannot respond to another impulse
Relative Refractory Period
Part of ST and T wave
May or may not respond to a premature impulse
Can skew EKG
Vulnerable period
Majority of T wave
Cells are still repolarizing
Premature impulses can be dangerous and can deteriorate patient into V-tach or V-fib (“R on T”)
p wave
Atrial depolarization
Symmetrical and rounded
Before QRS
increases with atrial hypertrophy
QRS complex
From the beginning of the QRS to the end
The composite of electricity in the ventricular septum and the right and left ventricles
Represents ventricular depolarization
Normal 0.06 to 0.10 seconds
1 ½ to 2 ½ boxes
PR interval
From the beginning of the P wave and goes to the first deflection of the QRS complex
Impulse activity in the heart before it gets to the ventricle
Normal 0.12 – 0.20 seconds
3-5 boxes
Represents the length of time required for SAN stimulation, atrial depolarization and conduction through the AVN before ventricular depolarization
ST segment
Represents early repolarization and lasts from the end of the QRS complex to the beginning of the T wave
The ST segment is normally isoelectric
At baseline
If it above or below the isoelectric line, it may be a sign of cardiac ischemia/infarction
Below – ischemia
Above - infarction
T wave
Represents ventricular repolarization
It follows the QRS complex and is usually the same direction as the QRS complex
T wave is asymmetric
QT interval
Beginning of the QRS to the end of the T wave – measures ventricular depolarization and repolarization
If the QT interval becomes prolonged, the patient may be at risk for a lethal ventricular dysrhythmia called torsades de pointes (V-tach)
0.45 seconds is prolonged
normal sinus rhythm
Definition – the electrical impulse starts in the SA node
Regular rate and rhythm
Impulse travels through the normal conduction pathway
Rhythm: Regular
Rate: 60-100 bpm
Atrial conduction – 1 p wave before each QRS, consistent in shape
AV conduction – PR interval 0.12-0.20 seconds
Ventricular conduction – QRS 0.06-0.10 seconds
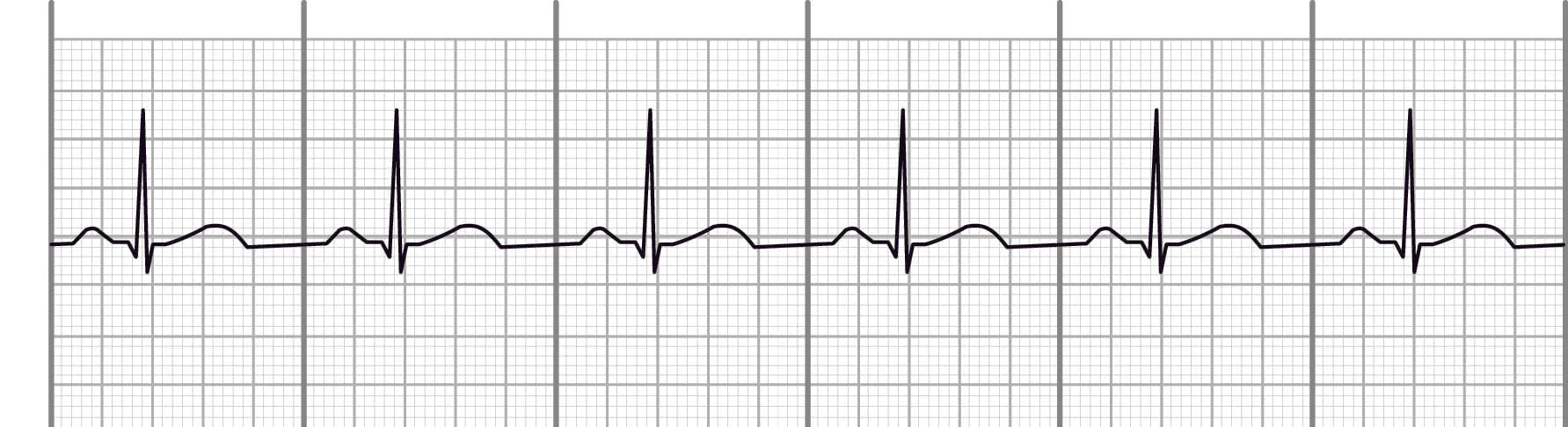
sinus tachy
Definition – occurs when the SAN creates an impulse at a faster than normal rate
Criteria:
Rhythm - Regular
Rate > 100 bpm
AV conduction – PR interval 0.12-0.20 seconds
Ventricular conduction – QRS 0.06-0.10 seconds
Causes:
Physiologic or psychological stress, blood loss, anemia, shock, hypovolemia, heart failure, pain, hyper-metabolic states, fever, exercise, anxiety, hypoxia, hypotension, medications, infection, hyperthyroid, stimulants, illicit drugs
Treatments
Treat the underlying cause
monitor patient
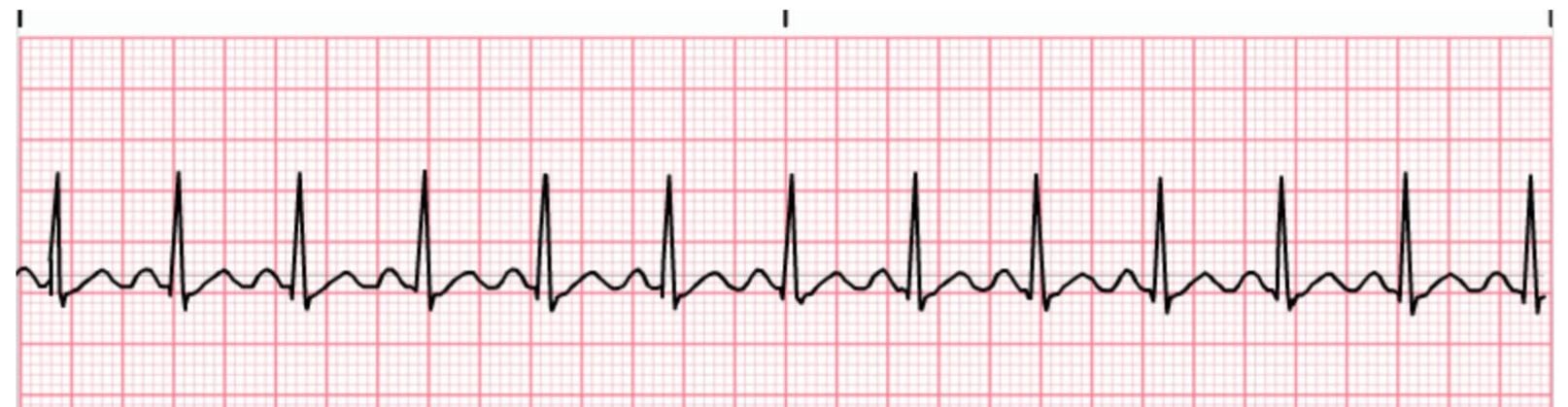
sinus brady
Definition – occurs when the SAN creates an impulse at a slower than normal rate
Criteria
Rhythm - Regular
Rate < 60 bpm
AV conduction – PR interval 0.12-0.20 seconds
Ventricular conduction – QRS 0.06-0.10 seconds
Causes – decreased metabolic needs, athletic training, hypothyroidism, vagal stimulation, medications, idiopathic SAN dysfunction, increased ICP, MI
Treatments
Monitor the patient
Treat only if symptomatic
Treat underlying cause
Atropine if caused by a vagal mechanism
Blocks parasympathetic nervous system
Unresponsive to Atropine
Transcutaneous pacemaker, dopamine, epinephrine
Transvenous pacemaker
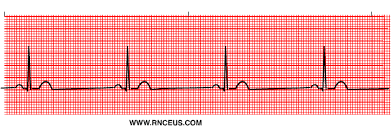
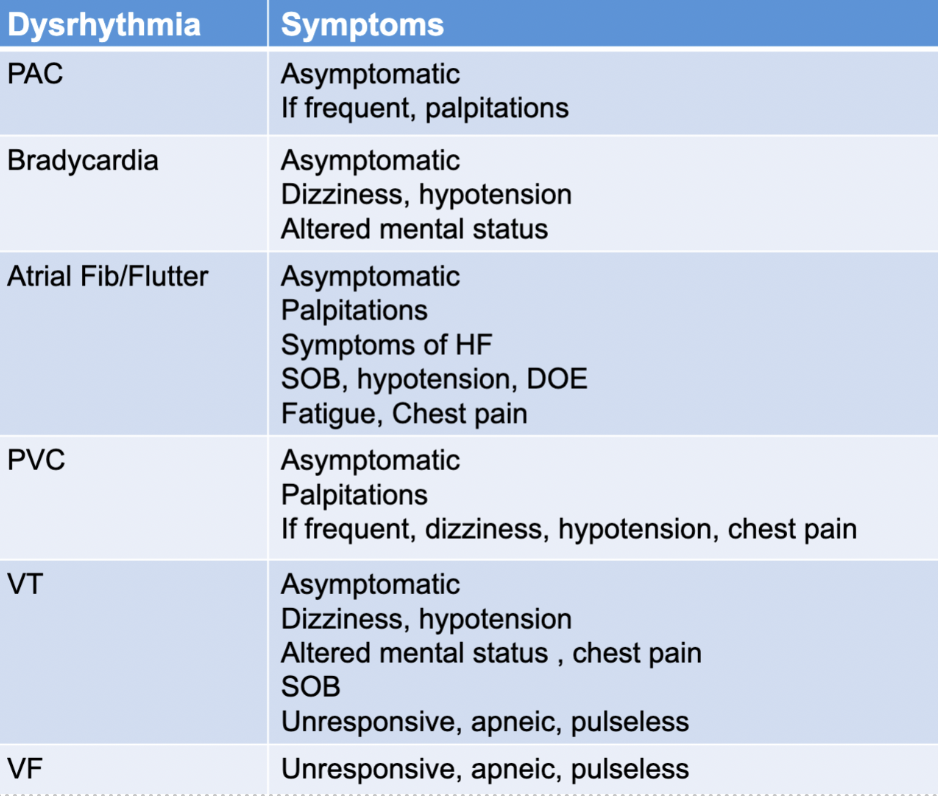
PAC
Definition – a premature, ectopic electrical impulse discharges in the atrium before the next normal impulse of the SA node
Ectopic beat – a pacemaker site outside of the SAN
Lots of ectopy = lots of irregularities
Complex comes in early
Criteria
Rhythm – regular except where the premature beat occurs
Rate – is the rate of the underlying rhythm
Atrial conduction – P wave before the ectopic beat is different from the p waves of the underlying rhythm
Causes – caffeine, alcohol, nicotine, stretched atrial myocardium, HF, anxiety, hypokalemia, hyper-metabolic states, atrial ischemia, medications
PACs are common in normal, healthy hearts
Treatment
No treatment is generally needed
Assess the patient
Remove causing agent
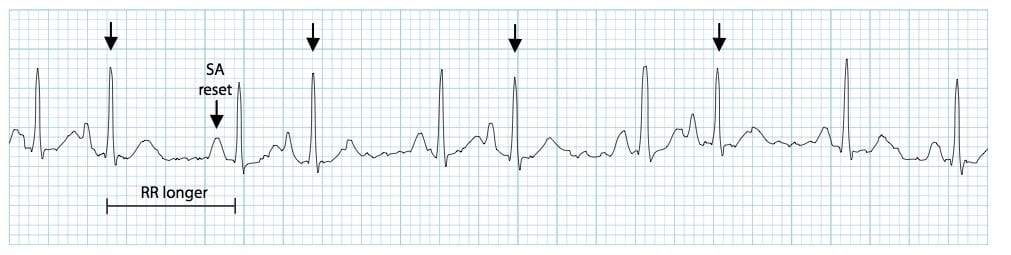
a flutter
Definition – atria are depolarized at rates of 250-400 times per minute
Caused by a reentry circuit
Not all the atrial impulses are conducted into the ventricle, causing a therapeutic block at the AV node
Criteria
Atrial rate is between 250-400
Ventricular rate variable
Atrial rhythm is regular
Ventricular rhythm can be either regular or irregular, depending on the conduction pattern
“P” waves produce a saw-tooth pattern called flutter waves
Causes
Usually associated with underlying cardiac disease
Long standing hypertension, cardiomyopathy, CAD, hypoxia, heart failure, valvular disease, SAN damage, post open heart surgery, digitalis toxicity
Blood stasis and thromboembolisms may occur due to lack of atrial contraction
Treatment
Control heart rate
Diltiazem, beta blockers, digitalis
Convert to NSR
Amiodarone
Cardioversion
Onto the r wave because it is the absolute refractory period
Vagal Maneuvers
Carotid sinus massage, Cough
Anticoagulation
when patient is unstable
Restless
Diaphoretic
Low BP
Chest pain
SOB
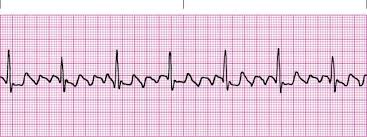
afib
Definition –
Uncoordinated atrial electrical activation from multiple areas in the atria
Results in a rapid, disorganized, quivering, fibrillating atria
The ventricular rate is dependent on the ability of the AV node to conduct the atrial impulses
Lack of atrial contraction increases the risk of thromboembolism formation
Characteristics
No P waves
EKG baseline is irregular and undulating
Ventricular rhythm is irregular and no pattern to the irregularity “Irregular, Irregularity”
Clinical Significance
Loss of atrial systolic volume “atrial kick”
The last bit of squeeze increases preload
Loss of AV synchrony
Reduced cardiac output
Increased potential for thromboembolism
Causes
Advanced heart disease, long standing hypertension, atherosclerosis, rheumatic heart disease, MI, HF, valve disease, pulmonary disease, after cardiac surgery, thyrotoxicosis, congenital heart disease
Rarely occurs in individuals with no functional heart disease
Goal
Decrease ventricular rate
Convert to NSR
Prevent thromboembolism
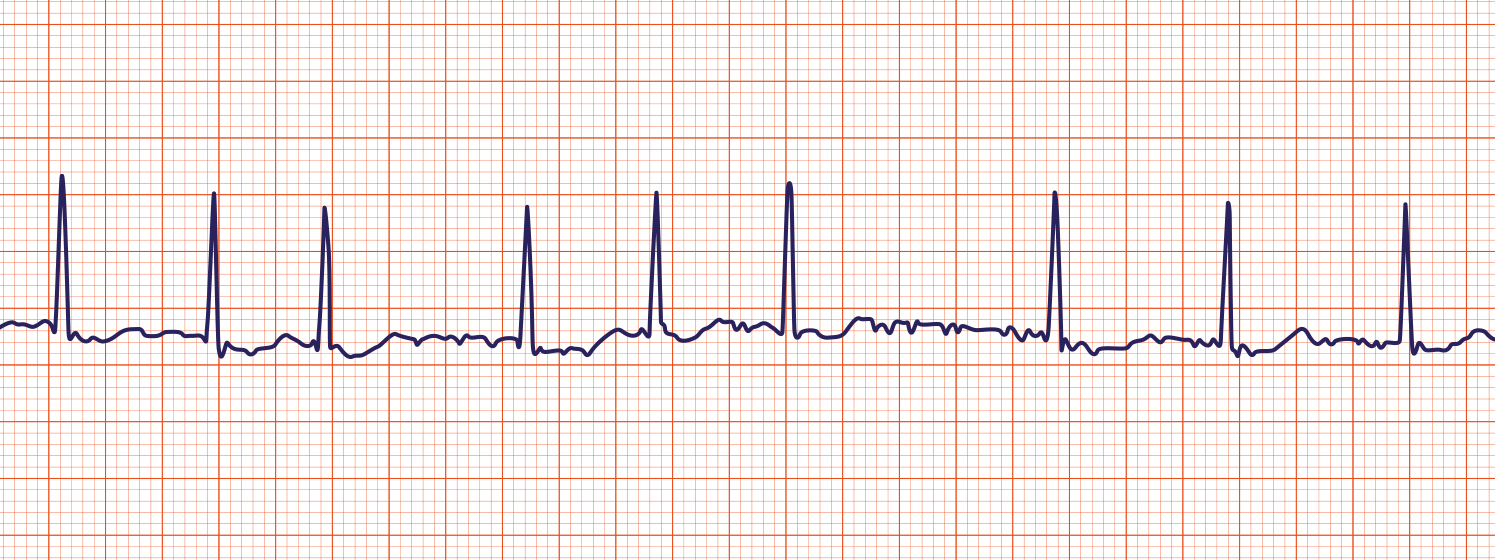
afib treatment and meds
Medications for rate control
Calcium channel blocker – diltiazem
Continuous infusion
Beta blockers
Just for rate control
Digoxin
Medications for conversion
Amiodarone
Loading dose and then PO
Dofetilide - Tikosyn
Flecanide – Tamboco
Thromboembolism in Afib
Origin is frequently the left atrial appendage
Left atrial appendage closure
Watchman
Most patients require some antithrombotic therapy
Antiplatelet agents - Aspirin
Antithrombotic medications
Anticoagulants
Warfarin
Factor Xa inhibitors
Dabigatran
Rivaroxaban
Apixaban
Treatment
Cardioversion if unstable
Cardioversion – synchronized defibrillation
Shock on the R because absolute refractory period
Invasive treatments
Maze procedure
Cut the atrial tissue to form scar tissue to stop the electrical impulses form forming
Pulmonary vein ablation
Some patients remain chronically in Afib
PVC
Definition
Premature ectopic impulse that originates in the ventricle
Conducted through the ventricles before the next normal sinus impulse
These can occur in healthy people
Characteristics
Ventricular complex that comes in early
QRS is wide, shape is bizarre and there are
ST and T wave changes
PVC’s make the rhythm irregular
Causes – caffeine, nicotine, alcohol, cardiac ischemia, heart failure, digitalis toxicity, hypoxia, acidosis, electrolyte imbalances, especially hypokalemia, obstructive sleep apnea
Multifocal
More than one area firing prematurely
PVC are asymmetric
Treatment
Infrequent – no treatment needed
Remove causing agent
Amiodarone, lidocaine
Amio is second line
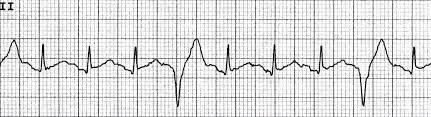
Vtach
Definition
3 or more PVCs in a row,
Rate exceeding 100 bpm
This is an emergency and can be a life-threatening dysrhythmia
Characteristics
Ventricular rate is >100 bpm
Rhythm is usually regular
QRS complexes are wide
No P waves present
Causes – MI, ischemia, hypoxia, electrolyte imbalance, CAD, acidosis, digitalis toxicity, drug intoxications, HF, cardiomyopathy, valve disease
Treatment
Pulse present
Stable – antiarrhythmic drugs, amiodarone
Unstable but conscious
Sedate
Cardioversion
Antiarrhythmic drugs
Pulseless – defibrillation
Unsynched
CPR
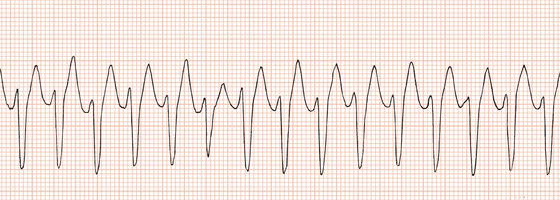
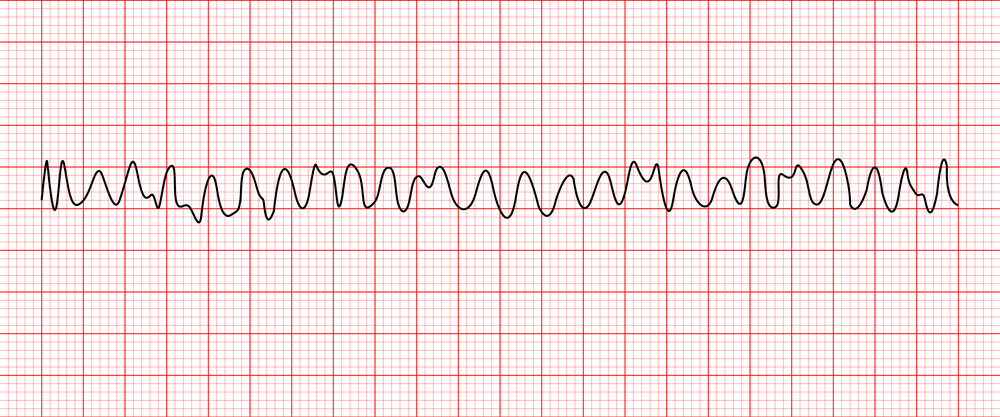
vfib
Definition
Rapid, disorganized ventricular rhythm that causes ineffective quivering and pumping of the ventricles
Life-threatening dysrhythmia
Characteristics
No atrial activity seen
Ventricular rhythm is extremely irregular and chaotic with no pattern
QRS shape is irregular with undulating waves without recognizable QRS complexes
Causes
The most common cause of cardiac arrest
CAD, ischemia, MI, HF hypoxia, acidosis, electrolyte imbalances, valve disease
Treatment
CPR, defibrillation, Epinephrine, amiodarone, vasopressin, lidocaine, sodium bicarbonate
dysrhythmia symptoms
cardioversion and defribrillator
Cardioversion
Delivery of a timed or synchronized shock to terminate a tachy-dysrhythmia
Shock is synchronized with the “R” wave
Shock delivered during absolute refractory period
Defibrillation
Unsynchronized electrical shock administered in an emergency situation to terminate a life-threatening dysrhythmia
Causes depolarization of a critical mass of myocardial cell all at once;
Sinus node will then recapture its role as the primary pacemake
causes of acquired valve disease
Rheumatic heart disease
caused by strep
Infective endocarditis
Degenerative or age related
Myocardial infarction
Marfan’s syndrome
Trauma
Lupus
Scleroderma
stenosis
Restricts the forward flow of blood because the valve is unable to fully open
Results in:
Increased the workload of the heart (increases afterload)
Hypertrophy of the of the chamber pumping against the stenotic valve
Level 1 murmur
regurg
Valve does not close completely and permits the backward flow of blood
Results in:
Increased blood volume in the cardiac chamber receiving the backflow of blood
Over time, chamber receiving regurgitant blood flow will become dilated
stenotic valve
Results in hypertrophy of atria -> Afib
regurgitant/incompetent valve
Ventricle contracts and blood flows backwards into atria resulting in hypertrophy -> afib and a flutter
Over time leads to ventricular hypertrophy as well
Murmur grading

aortic stenosis
Definition – an obstruction of blood flow from the left ventricle into the aorta during systole
Systolic murmur
Caused by a narrowing of the valve opening
AS causes an increase LV workload leading to LV hypertrophy
Over time, severe AS can cause:
A decrease in LV function
Resulting in heart failure (right sided and left sided)
Risk factors
Congenital bicuspid valve
Degenerative stenosis
Age
Diabetes
Elevated cholesterol
Hypertension
Smokin
Clinical Manifestations
Exertional dyspnea
Exercise intolerance
Fatigue
Angina
Syncope – fixed cardiac output
Cannot adjust CO
Systolic ejection murmur
Heart failure
Slower heart rate – takes longer for LV to eject blood
aortic regurg
Definition:
Backflow of blood from the aorta into the LV during diastole -> diastolic murmur
Due to ineffective closure of the aortic cusps
LVED volume increases
Over time causes dilation of the LV and subsequent heart failure
Lowers diastolic BP or widened pulse pressure because of backflow of blood
Risk Factors
Infective or rheumatic endocarditis
Congenital malformations
Dissection aortic aneurysm
Chest trauma
Marfans
Clinical Manifestations
Fatigue
Dyspnea on exertion
Palpitations
Dizziness
Sensation of a forceful heartbeat
Angina – due to drop in diastolic pressure – decreases coronary artery perfusion
Heart failure
Diastolic murmur – high pitched blowing sound
aortic valve disorder treatments
Aortic Stenosis
Echo surveillance
Medications
ACE inhibitors – reduce afterload
Statins – some evidence to suggest they slow disease progression
Surgery – Aortic valve replacement
Aortic balloon valvuloplasty
High mortality
TAVR – trans catheter aortic valve replacement
Worry about hemorrhage with arterial stick
Contrast dye can damage kidneys
Aortic Regurgitation
Echo surveillance
Medications – afterload reduction
Vasodilators, - ACE inhibitors, calcium channel blockers
Sodium restriction
Surgery – Aortic valve replacement
Surgery should be done before symptoms develop
Operative mortality is 3%
Patient with marked cardiac enlargement and LV dysfunction - mortality rate 10%
mitral stenosis
Definition
Obstruction of LV filling due to a narrowing/incomplete opening of the orifice of the mitral valve
MV is open during diastole to allow blood to flow from the left atrium to the left ventricle
Patho
Increased work of the left atrial causes hypertrophy
Left atrial hypertrophy increases the risk of developing A fib
Patients who develop A fib can become very symptomatic because they rely on atrial systolic volume to contribute to left ventricular end-diastolic volume
Atrial systolic volume contributes to 10 % of EDV
Blood backs up into the lungs
Risk Factors
Streptococcal infections
Congenital valve abnormality
Clinical Manifestations
Dyspnea on exertion
Fatigue
Palpitations and chest pain
Left atrial enlargement - increases risk of A fib and thrombus formation
Orthopnea, paroxysmal nocturnal dyspnea
Pulmonary edema
Heart failure
Diastolic murmur – low frequency rumble
mitral regurg
Definition – backflow of blood from the LV to the LA during systole due to incomplete closure of MV
Can be acute or chronic
Volume overload leads to ventricular dilation
Causes progressive LA dilation and RV dysfunction and pulmonary hypertension
Risk Factors – HF, endocarditis, mitral valve prolapse, acute MI
Volume overload occurs in the
Left atrium
Left ventricle
Leads to ventricular dilation
Causes progressive
LA dilation
RV dysfunction and
Pulmonary hypertension
Clinical Manifestations
Tachycardia
Fatigue, weakness
Exertional dyspnea
Orthopnea
Increased risk of atrial fibrillation
Signs of left sided heart failure
Pulmonary edema and congestion
Later - right sided heart failure
Pansystolic or holosystolic murmur
The entire time during systole
treatments for mitral disorders
Mitral Stenosis
Echo surveillance
Control HR – beta blockers
Treat A fib
Anticoagulate for A fib
Mitral valvuloplasty
Highly successful with low rate of restenosis
Surgery
Repair or replace valve
Mitral Regurgitation
Echo surveillance
Medications
Vasodilators – hydralazine, ACEI
Rate control for A fib – beta blockers, calcium channel blockers
Anticoagulate for a fib/a flutter
Diuretics for volume overload
Surgery
Valve repair
Valve replacement
types of valves
Tissue Valves
Don’t last as long as mechanical valves
Don’t require lifelong anticoagulation
Mechanical Valves
Require lifelong anticoagulation
Generally last longer than tissue valves
valve selection criteria
Patient’s age
Patient’s size
Medical history
Ability to comply with a medical regime
Warfarin
If young female -> tissue because of pregnancy
If young in general -> mechanical bc lasts longer
prosthetic valve complications
Thrombus formation
Especially in the mitral position
Leaking around valve
Infective Endocarditis
Antibiotic prophylaxis prior to any dental procedures;
1 large dose of an antibiotic 1 hour before procedure (i.e. amoxicillin 2 grams 1 hour before procedure)
Degenerative changes in tissue valves
Complications associated with prolonged anticoagulation therapy
mitral valve prolapse
Definition – a type of mitral valve insufficiency that occurs when one or both of the mitral valve cusps billow into the atrium during systole
Physical Signs
Mid or late systolic click
Diagnosis
Echocardiogram TTE and/or TEE
Treatment
Beta blockers for palpitations
Avoid stimulants
If symptomatic, valve repair or replacement
infective endocarditis
Definition – an infection of the endocardial surface of the heart which may include one or more heart valves, the endocardium or a septal defect. Requires:
Endocardial injury
Bacteremia
Endocardial injury attracts platelets and fibrin
Bacteria adheres to the injured surface and produces a vegetation
Vegetation enlarges as the pathogen, more platelets and more fibrins are attracted to the site
Vegetation can breakoff and travel via the bloodstream to other organs
May cause stroke, sepsis, pericarditis, infarction of the lung, spleen, liver kidney and myocardium
Risk Factors
Recent dental procedures, poor dental health,
History of congenital heart disease or valvular disease
Long-term indwelling IV-line, hemodialysis
IVDA, indwelling urinary catheters prosthetic valves, implanted hardware
Diagnosis
Patient history and physical
Blood Cultures
Echocardiogram
IE manifestations and treatment
Manifestations
Fever, elevated WBC
Heart murmur
Roth spots - hemorrhages in the fundi of the eyes caused by emboli
Symptoms of heart failure due to valve malfunction
Stroke symptoms
Skin manifestations
Janeway lesions –small hemorrhages on palms or soles of feet
Osler nodes – small tender lesions on fingers or toe pads
Splinter hemorrhages -1-2 mm red-brown streaks on the nail bed due to small emboli
Treatment
6-week course of antibiotic therapy
Supportive Therapy
May require valve replacement surgery