Section 6 - Anti-thrombotic (Anti-coagulant) Therapy
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Thrombosis
Thrombus formation under pathological conditions into otherwise normal vessels
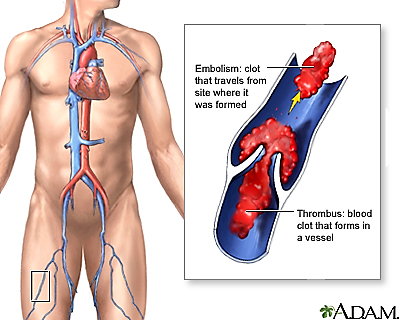
Thrombus
A blood Clot that forms in a vessel
Thrombi
Solid masses formed from blood components (platelets, fibrin, RBCs) within intact blood vessels or the heart.
Often in response to abnormal hemostasis or endothelial injury.
Can obstruct blood flow or embolize.
Embolus
Detached intravascular mass (thrombus, fat, air, etc.) that travels through circulation and lodges in a distant vessel.
potentially causing obstruction and ischemia.
Emboli
Plural of embolus
multiple intravascular particles (e.g., thrombi, fat, air, tumor) that travel through blood and obstruct distant vessels.
often leading to tissue ischemia or infarction.
Mechanism of DIC
Trigger: endothelial damage or tissue thromboplastin release
• Initiates systemic activation of coagulation cascade
• Widespread microvascular thrombi form
• Platelets and clotting factors consumed
• ↓ Fibrinogen, thrombocytopenia
• Ischemia and infarction from blocked vessels
• Bleeding from lack of clotting components
• Organ failure from hypoxia and fibrin deposition
Causes of DIC (General)
• Bacterial infections → cytokines damage endothelium → expose tissue factor → initiate clotting
• Intravascular parasites → vessel wall trauma → activates intrinsic pathway
• Surgery → tissue disruption → massive thromboplastin release → triggers coagulation
• Major trauma → crush injuries release TF-rich material → widespread thrombin activation
• Casting of legs → prolonged stasis + pressure → endothelial injury → local clotting spreads
• Burns → widespread endothelial loss → exposes subendothelial collagen → triggers clotting
• Snake venom → direct procoagulant activity (thrombin-like enzymes) → uncontrolled fibrin deposition
• Acute Promyelocytic Leukemia (APL) → blasts release procoagulants (e.g., cancer procoagulant) → initiates systemic coagulation
Secondary Fibrinolysis: Complications of Pregnancy
• Dead fetus syndrome → retained fetal tissue releases thromboplastin → systemic clotting
• Amniotic fluid embolus → AF enters circulation → contains TF & procoagulants → activates clotting
• Abruption placentae → placental detachment releases large amounts of TF into maternal blood
• Preeclampsia → endothelial dysfunction and vasospasm → triggers clot formation
• Eclampsia → severe progression of preeclampsia → worsens endothelial injury
• HELLP syndrome → hemolysis + liver injury + low platelets → promotes DIC-like state
• ↑ Coagulation factors (VII, VIII, IX, XII, I) → hypercoagulable state → increases risk of widespread clotting
• ↓ Protein S → impairs anticoagulant control of thrombin → favors clot propagation
Signs of Secondary Fibrinolysis
• Oozing at IV sites, mucous membranes
• Bleeding from GI, urinary, and respiratory tracts
• Hematuria, hematemesis, epistaxis
• Diffuse bruising or petechiae
• Delayed clot formation or rebleeding
• ↑ Fibrin degradation products (e.g., D-dimer)
Anti-Thrombotics
• Used to limit or prevent clot formation
• Act by suppressing synthesis or function of hemostatic components
• Goal: prevent thrombosis while avoiding excessive bleeding (hemorrhage)
• Balance efficacy with bleeding risk management
Warfarin (Coumadin, Jantoven)
General
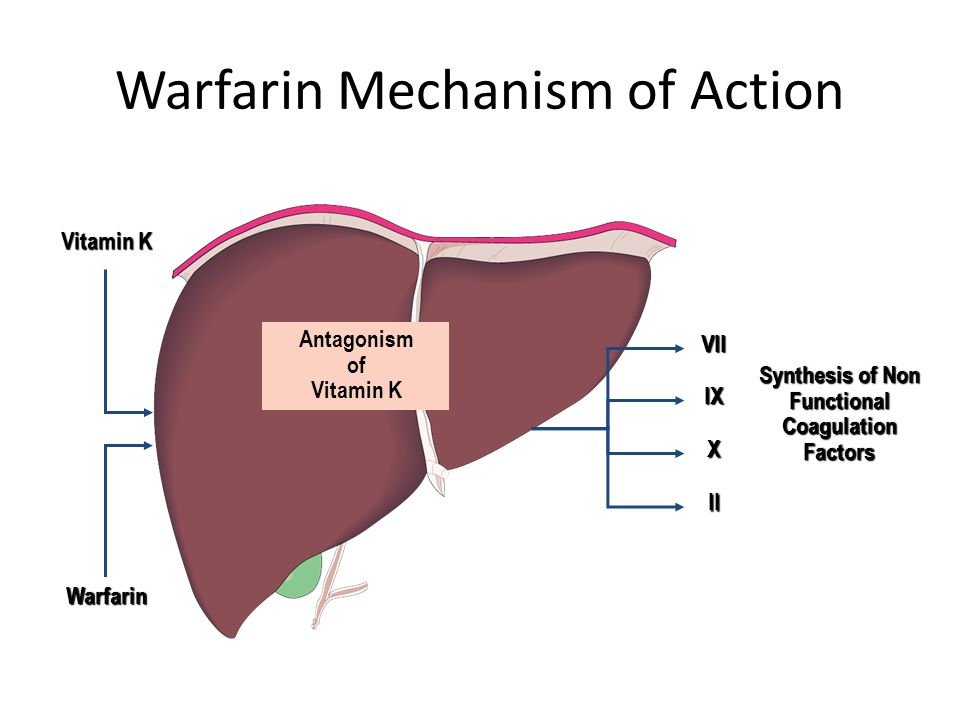
• Mechanism: Inhibits vitamin K epoxide reductase → prevents recycling of vitamin K → blocks γ-carboxylation of clotting factors II, VII, IX, X (and proteins C, S, Z) → produces nonfunctional factors
• Effect: Delays clot formation by reducing availability of functional vitamin K–dependent factors
• Monitoring: PT and INR (goal INR: 2–3); long half-life (~5 days)
• Caution: Interfered with by vitamin K–rich foods
Warfarin (Coumadin, Jantoven)
Uses
– Long-term anticoagulation (e.g., atrial fibrillation)
– Mechanical heart valves
– Treatment and prevention of DVT/PE
– Hypercoagulable states (e.g., protein C/S deficiency)
Heparin (UFH) – Mechanism of Action
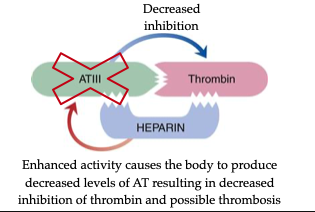
• Binds antithrombin (AT) → induces conformational change
• Enhances AT inhibition of IIa, Xa, IXa, XIa, XIIa, and plasmin
• Heparin is not consumed → acts as a cofactor repeatedly
• ↓ circulating AT with prolonged use (feedback inhibition)
Heparin (UFH) – Indications
• First-line anticoagulant for venous thrombosis, PE, acute coronary syndrome
• Used acutely, often followed by oral anticoagulants (e.g., warfarin)
• Administered IV or subQ; effects reversed with protamine sulfate
Heparin (UFH) – Monitoring & Risks
• Monitored using: APTT or Anti-Xa assay
• Expected therapeutic range:
– APTT: 1.5–2.5× the normal reference interval
– Anti-Xa: 0.3–0.7 IU/mL (if used)
• Monitor closely due to risk of Heparin-Induced Thrombocytopenia (HIT)
• Gradual tapering required to avoid rebound thrombosis due to ↓ antithrombin (AT)
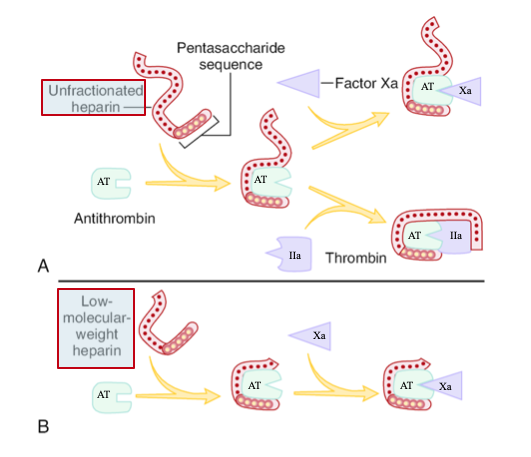
LMWH – Mechanism of Action
Derived from UFH via depolymerization
• Binds AT and preferentially inhibits factor Xa
• Less activity against thrombin (IIa) due to shorter chain length
• Longer half-life and more predictable response than UFH
LMWH – Indications
• Treatment and prevention of DVT, PE, post-surgical thrombosis
• Often used instead of UFH due to ease of dosing
• Administered subcutaneously, does not require routine monitoring
LMWH – Monitoring & Risks
• Monitored using: Chromogenic Anti-Xa assay (only when needed)
• Expected therapeutic range:
– Anti-Xa (twice-daily dosing): 0.6–1.0 IU/mL
– Anti-Xa (once-daily dosing): 1.0–2.0 IU/mL
• APTT is not useful for LMWH
• Typically does not cause HIT, but may cross-react with HIT antibodies in affected patients
DTIs – Mechanism of Action
Bind directly to active site of thrombin (IIa)
• Block thrombin's ability to convert fibrinogen → fibrin
• Inhibit both free and fibrin-bound thrombin
• Effectively neutralize thrombin without antithrombin
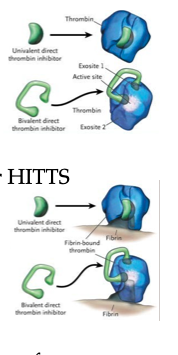
DTIs – Indications
Used as heparin alternatives, especially in HIT/HITTS
Drugs include:
– Argatroban (Novostan) – IV, half-life ~50 min
– Bivalirudin (Angiomax) – IV, half-life ~25 min
– Dabigatran (Pradaxa) – oral, half-life 10–18 hrs
DTIs – Monitoring & Dosing
• Monitored using: APTT
• Expected therapeutic range: 1.5–3× the mean baseline APTT
• No routine monitoring needed for dabigatran, but APTT or TT may be used if needed
• Reversal agent: Idarucizumab (for dabigatran only)
• Effective even when antithrombin (AT) levels are low or bypassed
Direct Factor Xa Inhibitors – Mechanism of Action
• Bind directly to and inhibit Factor Xa
• Prevent conversion of prothrombin (II) → thrombin (IIa)
• Block both free and clot-bound Xa
• Act independently of antithrombin
Direct Factor Xa Inhibitors – Indications
• Used in treatment and prevention of DVT, PE, stroke (AFib)
• Common agents: Rivaroxaban, Apixaban, Edoxaban
• Oral administration; do not require routine monitoring
Direct Factor Xa Inhibitors – Interference & Monitoring
• Drugs: Rivaroxaban, Apixaban, Edoxaban
• Monitoring test (if needed): Drug-specific chromogenic Anti-Xa assay
• Expected therapeutic range (approximate, varies by drug/dose/timing):
– Rivaroxaban: Peak Anti-Xa = 100–300 ng/mL
– Apixaban: Peak Anti-Xa = 100–200 ng/mL
• Routine monitoring not required
• Interfere with PT and APTT → false prolongation
• May cause false ↓ in factor assays and false ↑ in Protein C/S, AT, LA assays
• Do not interfere with immunoassays
Anti-Platelets – Mechanism of Action (Aspirin & NSAIDs)
Inhibit cyclooxygenase (COX) enzyme
• ↓ synthesis of Thromboxane A₂ (TXA₂)
• TXA₂ normally promotes platelet aggregation & vasoconstriction
• Result: ↓ platelet aggregation, ↓ clot formation
• Irreversible (aspirin), reversible (other NSAIDs)
Anti-Platelet Agents – Drug Classes & Names
• ADP receptor (P2Y₁₂) inhibitors
– Clopidogrel (Plavix)
– Ticagrelor (Brilinta)
– Prasugrel (Effient)
• Glycoprotein IIb/IIIa inhibitors
– Tirofiban (Aggrastat)
– Abciximab (ReoPro)
– Eptifibatide (Integrilin)
• PAR-1 antagonist (thrombin-related aggregation)
– Vorapaxar (Zontivity)
• Phosphodiesterase inhibitor (↑ vasodilation, ↓ aggregation)
– Cilostazol (Pletal)
Anti-Platelets – Monitoring & Clinical Notes
• Effect measured with Platelet Function Analyzer (PFA-100)
• Expected result: Prolonged closure time indicates platelet inhibition
• Most agents target platelet aggregation phase
• Aspirin/NSAID effect: ↑ PFA-100 closure time, especially with collagen/epinephrine cartridges
• COX inhibition is unique to aspirin and NSAIDs
• PAR-1 inhibitors not emphasized for exam purposes
Thrombolytics – Mechanism of Action
• Serine proteases that convert plasminogen → plasmin
• Plasmin degrades fibrin and fibrinogen in thrombi
• Breaks down clots to restore blood flow (e.g., in stroke, PE)
• Examples:
– Fibrin non-specific: Streptokinase, Urokinase, Anistreplase
– Fibrin-specific: tPA, Alteplase, Reteplase, Lanoteplase, Tenecteplase
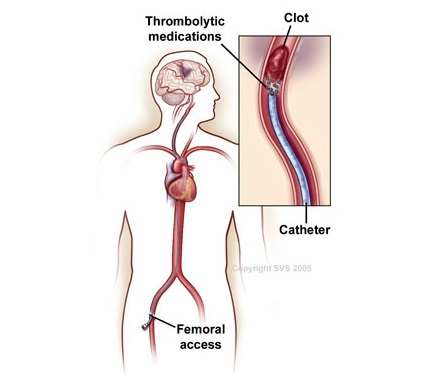
Thrombolytics – Indications
• Acute ischemic stroke (within 3–4.5 hrs)
• STEMI when PCI unavailable
• Massive PE with hemodynamic compromise
• Severe DVT or arterial thrombosis in select cases
• Catheter occlusions (e.g., central lines)
Thrombolytics – Monitoring & Therapeutic Effects
• Monitored using:
– Euglobulin clot lysis test (rarely used clinically)
– Fibrinogen levels (↓ indicates activity)
– PT, APTT, TT (↑ if fibrinolysis is systemic)
– FDPs, D-dimer (↑ from fibrin degradation)
• Expected results:
– ↓ fibrinogen
– ↑ D-dimer, FDPs
– ↑ PT/APTT/TT
• Common effect: hypofibrinogenemia
• Interferes with: most clotting assays due to fibrin degradation products
tPA (Tissue Plasminogen Activator)
• Fibrin-specific thrombolytic – activates plasminogen → plasmin at the clot site
• Dissolves fibrin-rich clots (e.g., embolic stroke, STEMI, PE)
• Includes drugs: Alteplase, Reteplase, Tenecteplase
• Most effective when given within 3–4.5 hours of stroke onset
• Monitored clinically; expect ↑ D-dimer, ↓ fibrinogen
• Major risk: intracranial hemorrhage
Mechanical Thrombectomy – Mechanism of Action
• Catheter-based procedure to physically remove a blood clot
• Stent retriever is inserted through a blood vessel (e.g., femoral artery)
• Device expands to trap and extract the clot
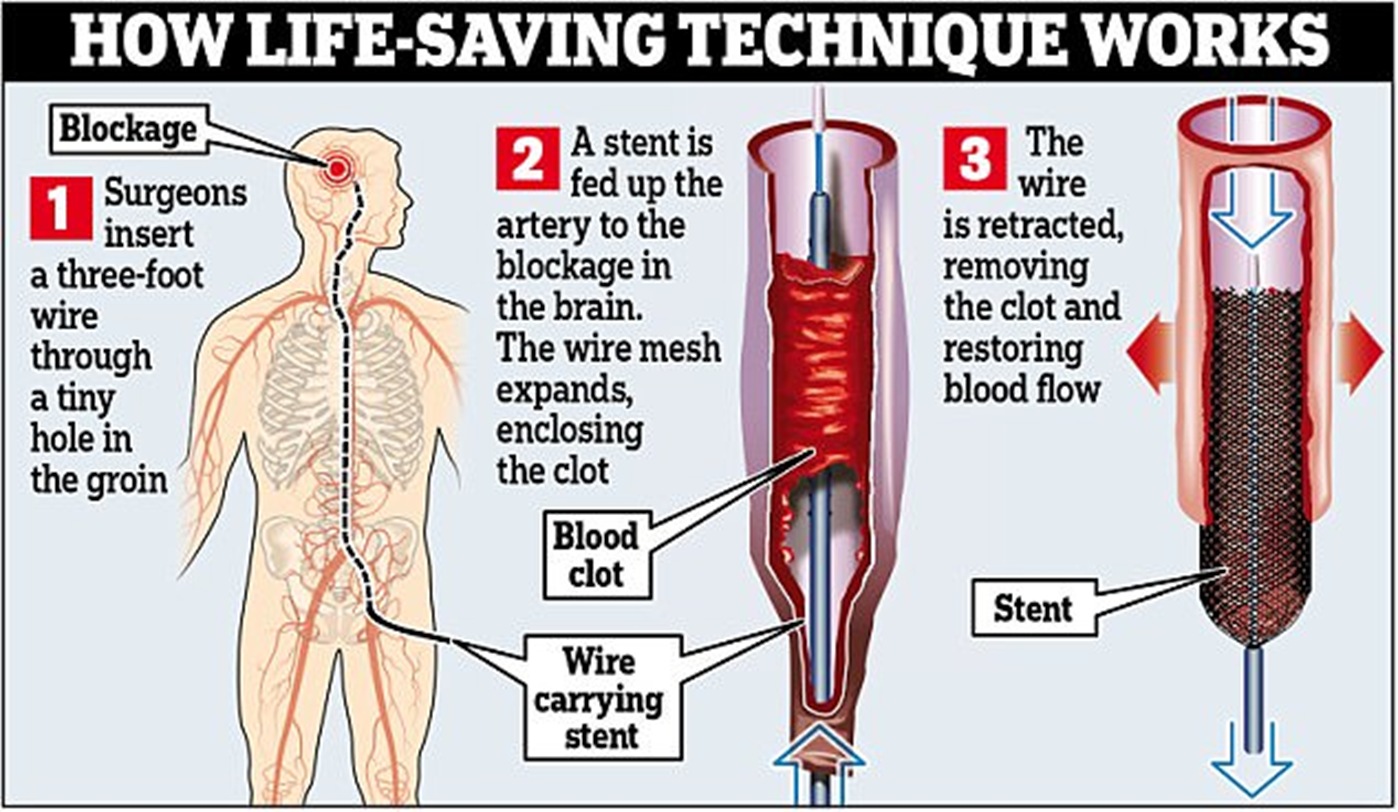
Mechanical Thrombectomy – Indications
• Acute ischemic stroke due to large vessel occlusion
• Typically used when:
– tPA is contraindicated
– tPA fails
– Patient presents within 6–24 hours of symptom onset (based on perfusion imaging)
• Sometimes used in peripheral artery disease or DVT (less common)
Watchman – Mechanism of Action
• A small implantable device placed in the left atrial appendage (LAA)
• Physically seals off the LAA, a common site of clot formation in atrial fibrillation
• Prevents clots from escaping into circulation and causing stroke
• Device endothelializes over time, eliminating need for lifelong anticoagulation
Watchman – Indications
• Patients with non-valvular atrial fibrillation at increased stroke risk
• Especially considered for patients who:
– Have contraindications to long-term anticoagulation
– Are at high risk of bleeding on oral anticoagulants
• FDA-approved alternative to lifelong anticoagulant therapy
• 96% of patients in trials stopped anticoagulants within 45 days post-implant