Chem 2380 Final Exam Reagents/Reactions
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
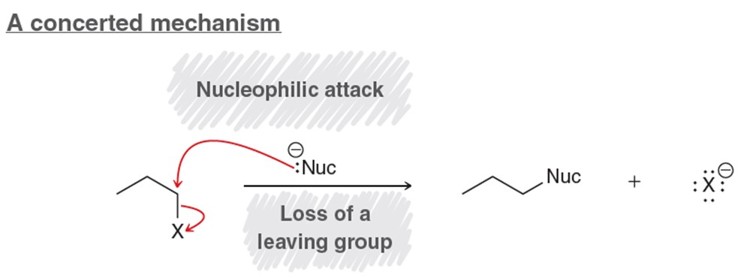
SN2 Reaction
Need a nucelophile and a good leaving group. Concerted mechanism. Mechanism is required
SN1 Reaction
Need a leaving group and a nucelophile. Forms a carbocation. Which will move to the more substituted carbon. Stepwise mechanism.

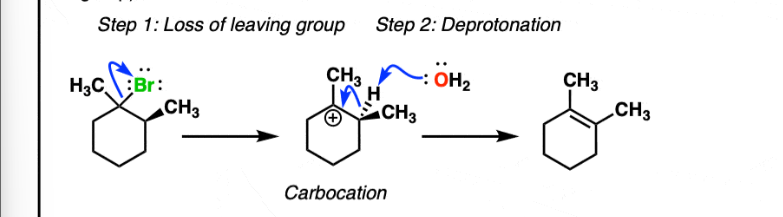
E1 Reaction
Does not require strong base, carbocation rearrangement is possible for a more stable carbocation.
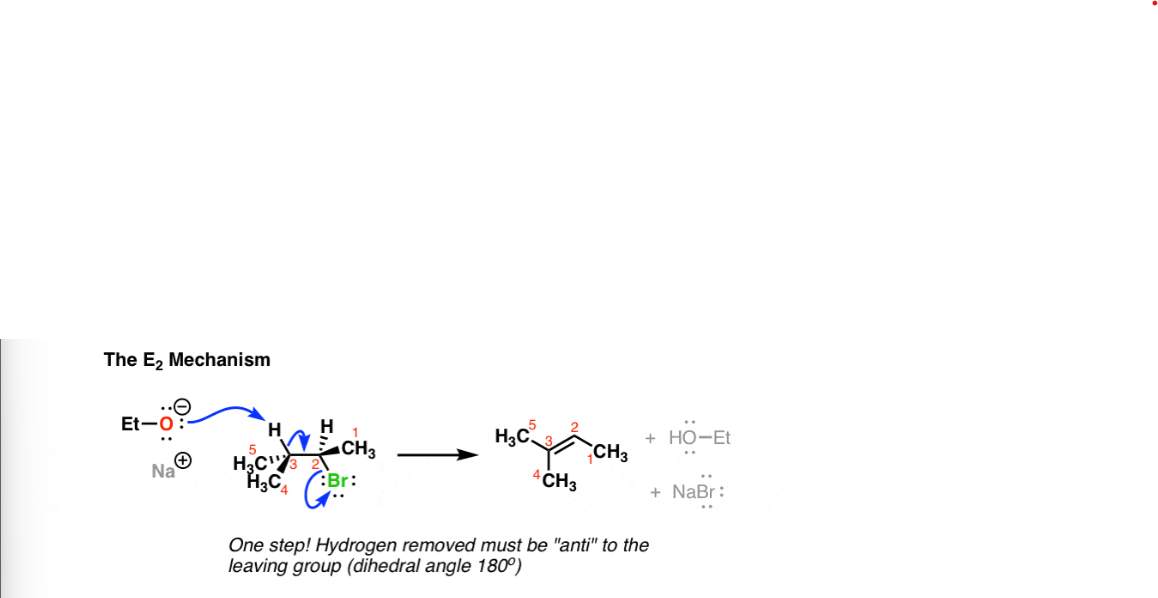
E2 Reaction
Requires a strong base, C—H bond must be 180 degrees to the C-LG bond.
Addition Reaction
Reactions of alkene. It is protonated by a strong acid. Forms a carbocation intermediate.
Catalytic Hydrogenation in the presence of a ketone/aldehyde
A reducing agent. H2 and a metal such as Pt, Pd, or Ni.

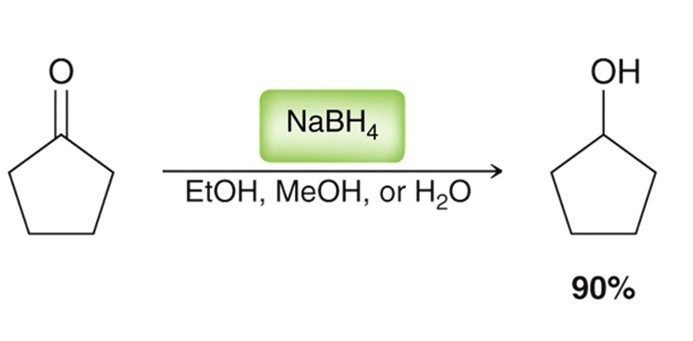
Sodium Borohydride in the presence of a ketone/aldehyde
A reducing agent. NaBH4 in a EtOH, MeOH, or H2O (protic solvent)
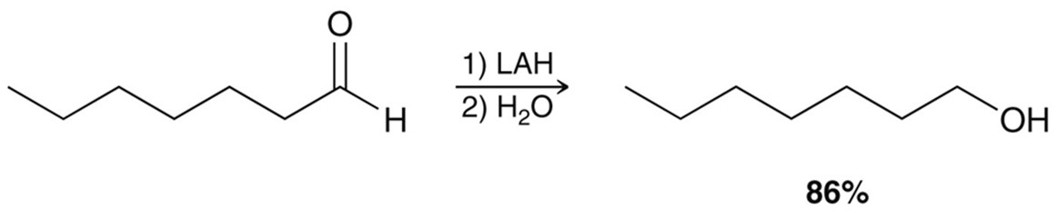
Lithium Aluminum Hydride (LAH) in the presence of a ketone/aldehyde
A reducing agent. LiAlH4 in a protic solvent (often water). Far more reactive than NaBH4
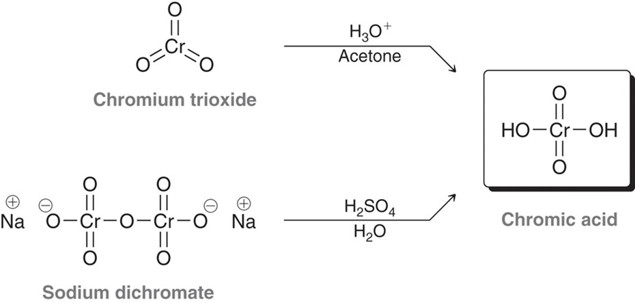
Chromic acid in the presence of a primary alcohol.
Oxidizing agent, CrO3 or Na2Cr2O7 in aqeuous acid. Will oxidize a primary alcohol to a carboxyilic acid.
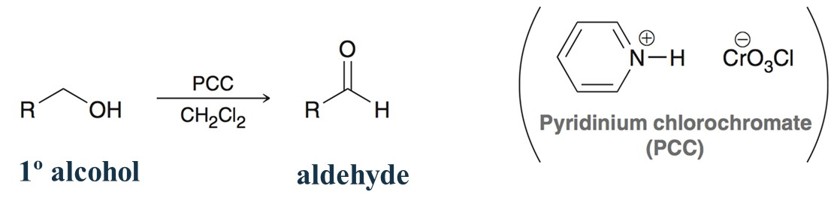
PCC in presence of a primary alcohol
Oxidizing agent. PCC or pyridinium cholorochromate used to produce an aldehyde.
PCC or Chromic acid in presence of secondary alcohol
Oxidizing agent. Will convert any secondary alcohol into a ketone.

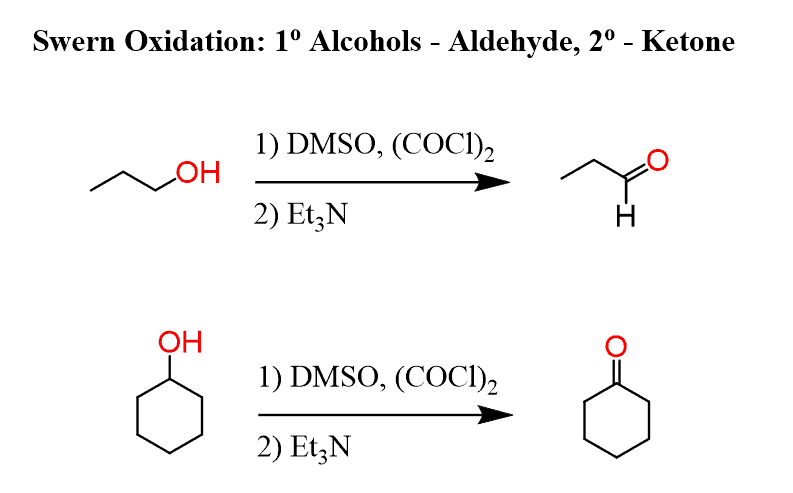
Swern Oxidation
Oxidation of primary alcohols to aldehydes and secondary alcohols to ketones. Using DMSO (dimethyl sulfoxide) and oxalyl chloride to form active oxidant. Et3N facilitates elimination to form pi bond. Mechanism is required.
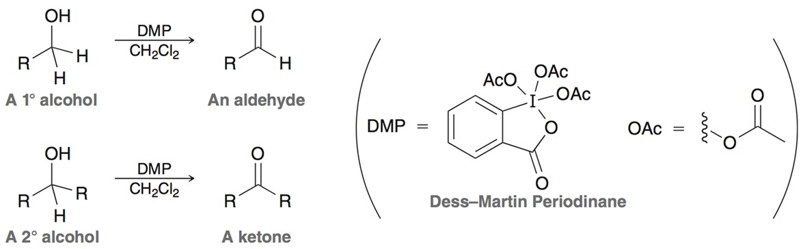
Dess-Martin Oxidation
Oxidizing reagent. DMP (Dess-Martin Periodane) oxidizes primary alcohols to aldehydes and secondary alcohols to ketones.
Grignard Reagent in the presence of a ketone/aldehyde.
Strong base and nucleophile. RMgX. Have to use aprotic solvents or protecting groups such as TMS.
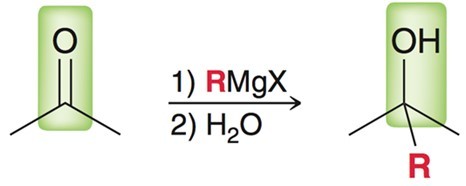
Preparation of conjugated diene via elimination
Using a sterically hindered strong base.
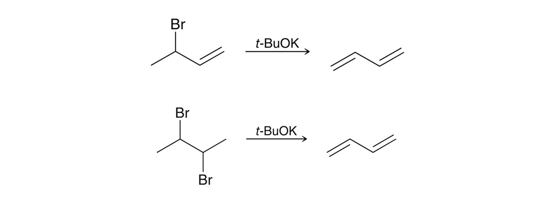
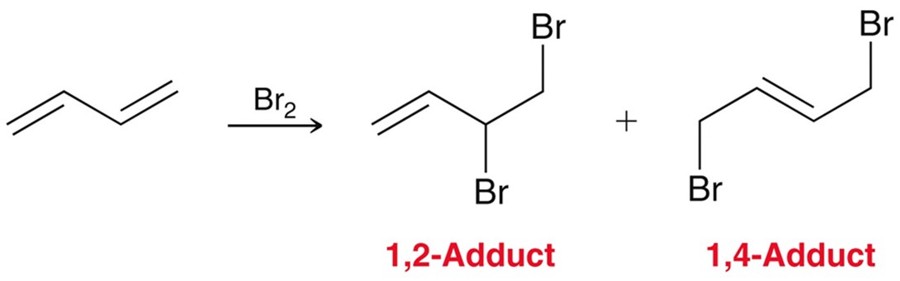
Electrophilic Addition to conjugated dienes
Adding X2 to a conjugated system resultings in the 1,2- and 1,4- addition products.
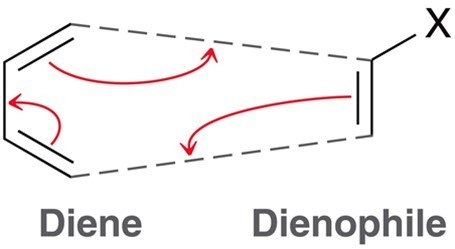
Diels-Alder Reaction
A conjugated system with an alkene form a cyclic or bicyclic system with a single pi bond or dimer. Dieneophile must posess an electron withdrawing group such as an R, OR, OH, or CN group. Diene must be in the s-cis conformation.
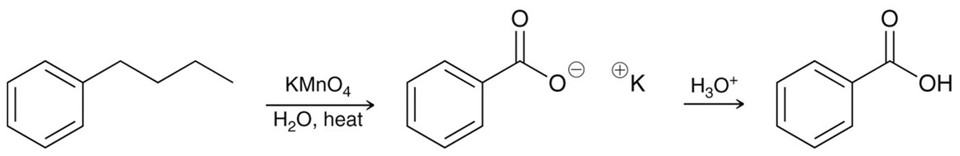
Permanganate in the presence benzylic positions
An oxidation reagent. KMnO4 with water and heat. Produces the deprotonated benzylic acid, add H3O+ to produce benzylic acid.
Free Radical Bromination
Benzylic positions can readily undergo free radical bromination using NBS and peroxides (ROOR)
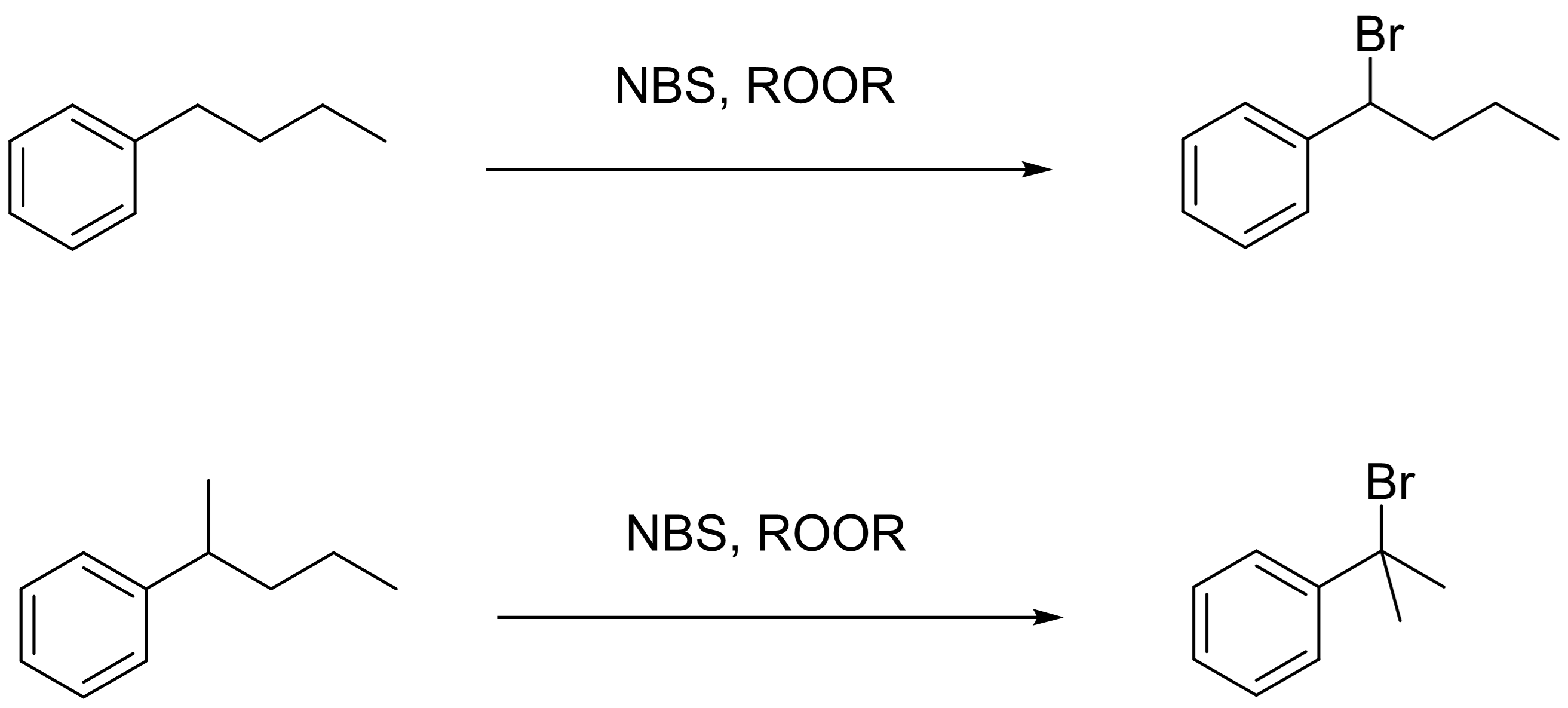
Alkenes in benzylic positions
Using H2 and Pt at 2 atm alkenes can be selectively hydrogenated in the presence of a benzene ring.

Reduction of Benzene
Using excess H2 and Ni at 100 atom. Benzene can be reduced to cyclohexane.

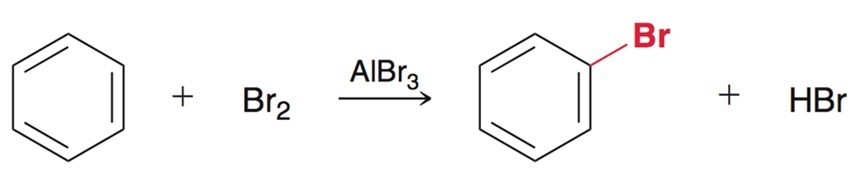
Electrophilic Aromatic Subsitution - Halogenation
Using X2 and a metal catalyst such as FeCl3/FeBr3/AlBr3. Mechanism required.
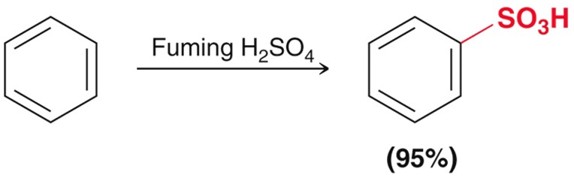
Electrophilic Aromatic Subsitution - Sulfonation
Using SO3 as the electrophile and H2SO4 as the catalyst. This whole reaction is equilibria.
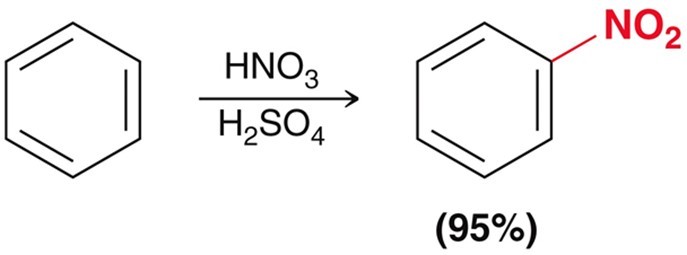
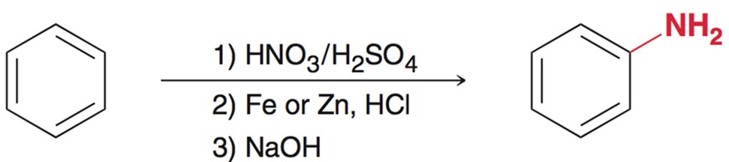
Electrophilic Aromatic Subsitution - Nitration
Using HNO3 and H2SO4 as the acid catalyst.
Formation of Aniline from Nitration
Using Fe or Zn, along with HCL and a strong base, usally NaOH forms Aniline a type of amine.
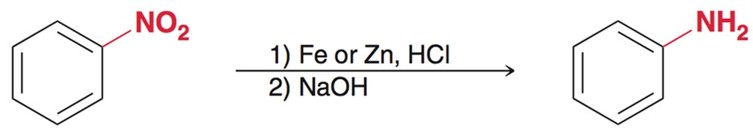
Benzene to Aniline
HNO3/ H2SO4
Fe or Zn, HCl
NaOH
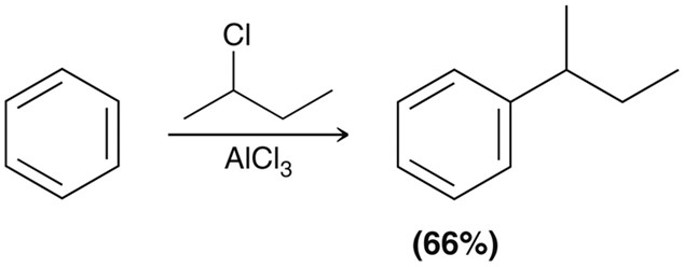
Friedel-Crafts Akylation
Using an akyl halide and AlCl3 as the acid catalyst produces a benzene with an akyl group. The carbocation acts as the electrophile. This reaction can also be done using a terminal alkene and H+ (a tertiary carbocation) or using cyclohexanol and BF3 where the OH-BF3 will act as a LG and form a secondary carbocation.
Friedel-Crafts Acylation
Using an acyl halogen (usually Cl) and AlCl3 to produce a aromatic ketone or acylbenzene.

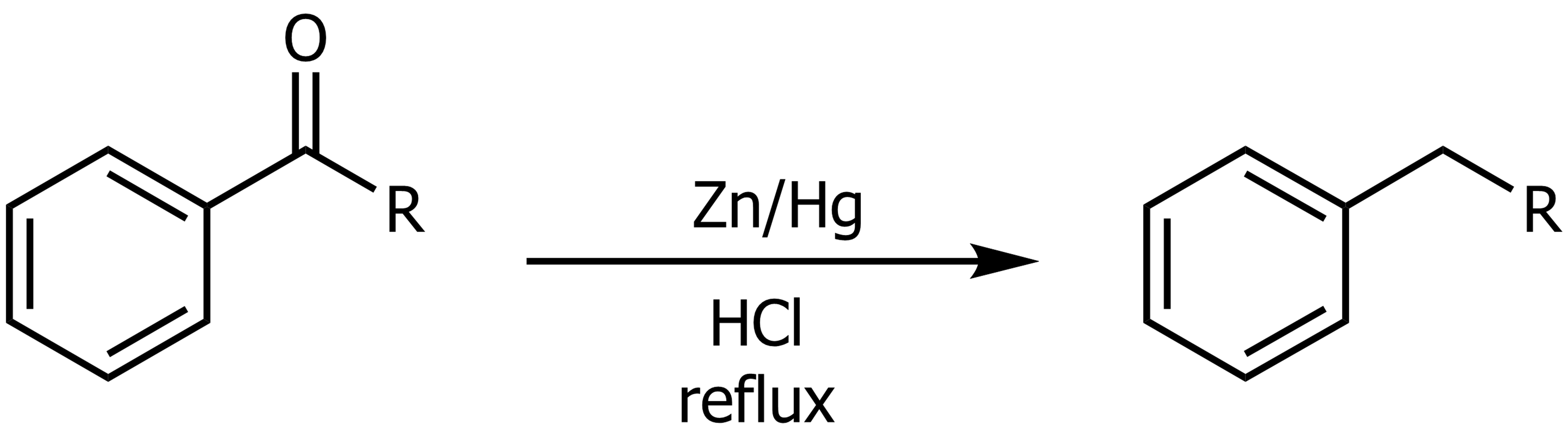
The Clemmensen Reduction
From an acylbenzene it can be reduced using Zn/Hg with HCl (reflux). Similar to Wolff-Kishner.
Nucleophilic Aromatic Substitution (SNAr)
A strong nucleophile attacks an electron poor benzene. The ring must possess a strong EWG and a good leaving group. This LG must be ortho/para to EWG.
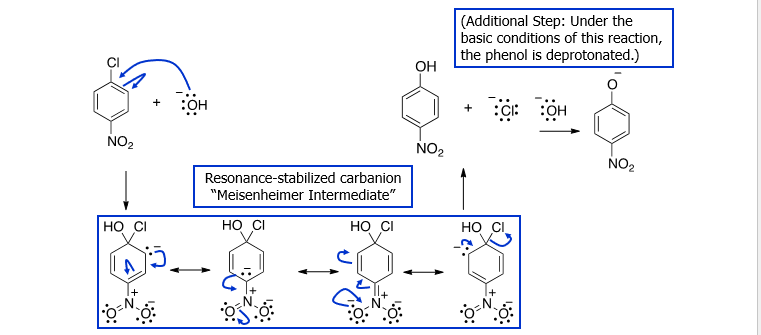
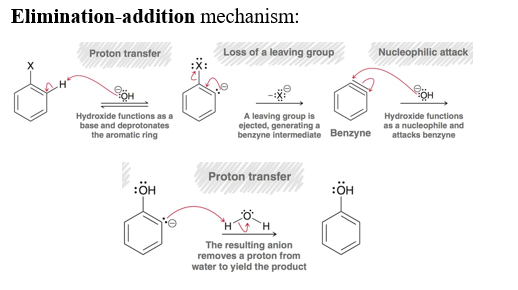
Elimination-Addition / Formation of Benzyne intermediate
Using NaNH2, NH3(l) and H3O+ in the presence of an chlorobenzene or another halobenzene. Produces it into Aniline. This forms the benzyne intermediate.
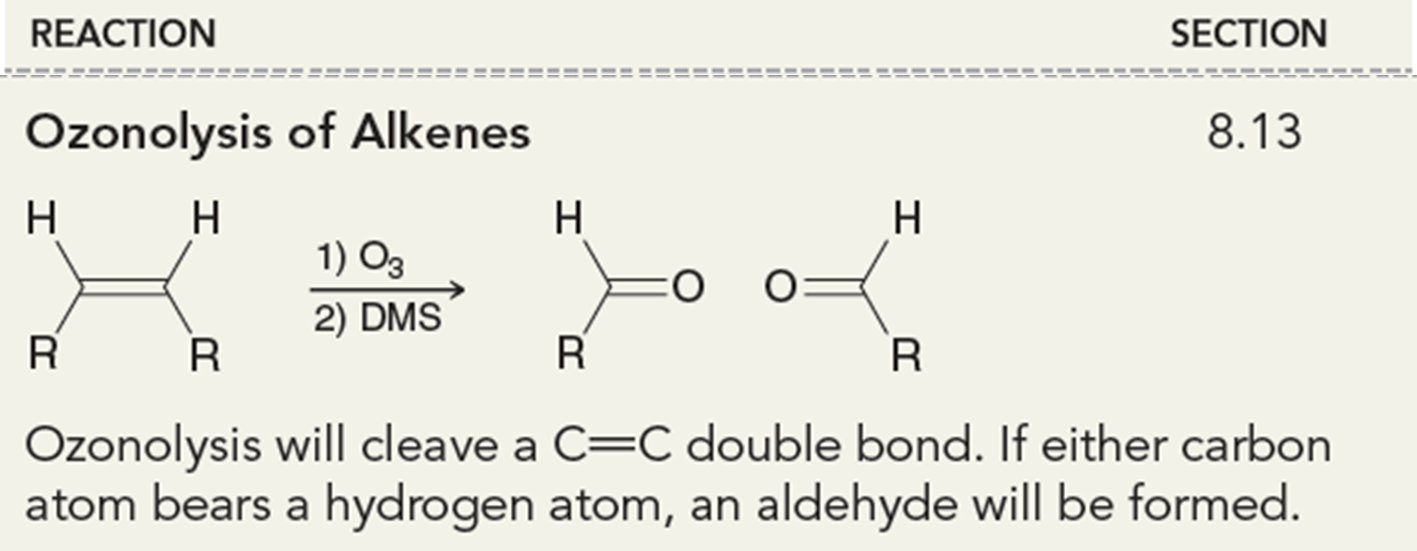
Ozonolysis of Alkenes
This will cleave a C=C double bond. This can form two aldehydes, two ketones, or one of each.
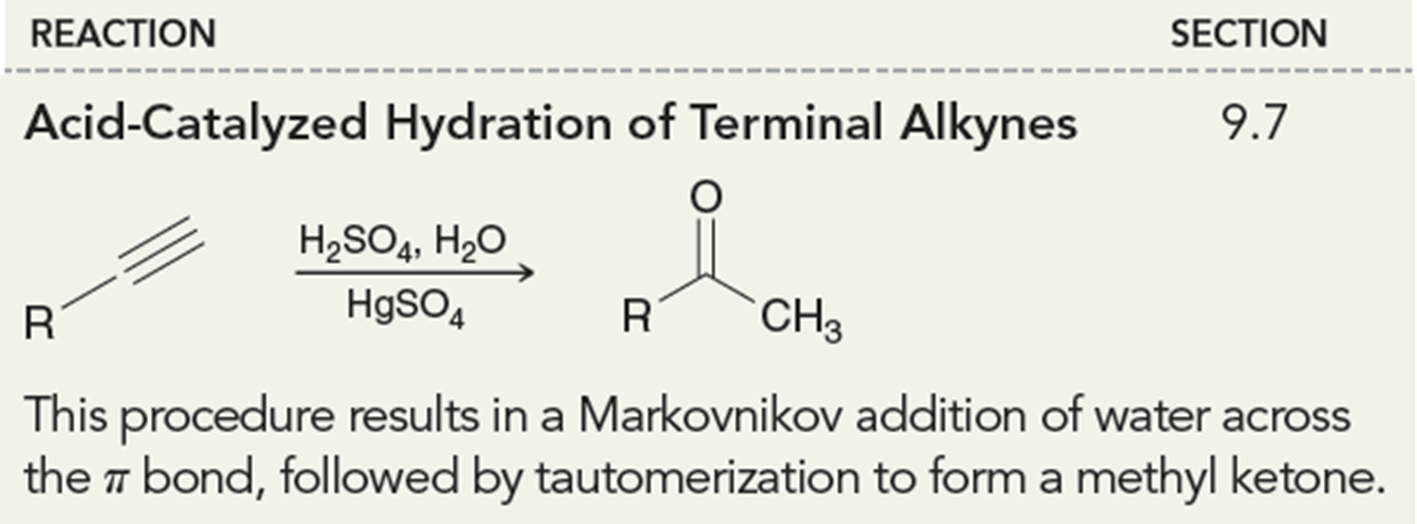
Acid-Catalyzed Hydration
In the presence of a terminal alkyne using H2SO4, H2O and HgSO4 this can produce a methyl ketone after tautomerization.
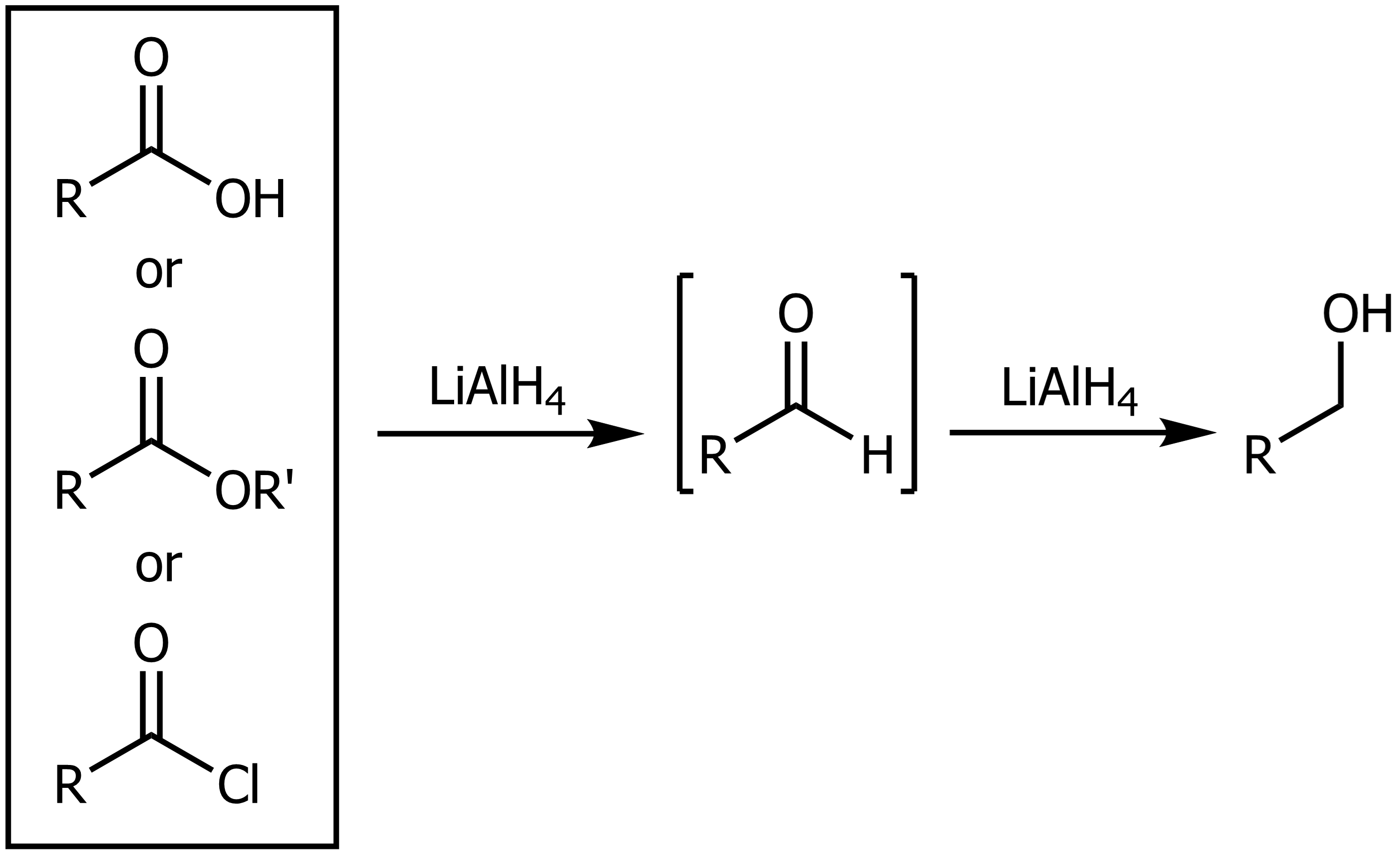
Reduction of Ester/Aldehydes and Acyl Chlorides
Using LAH or LiAlH4 on any of the above forms a primary alcohol.
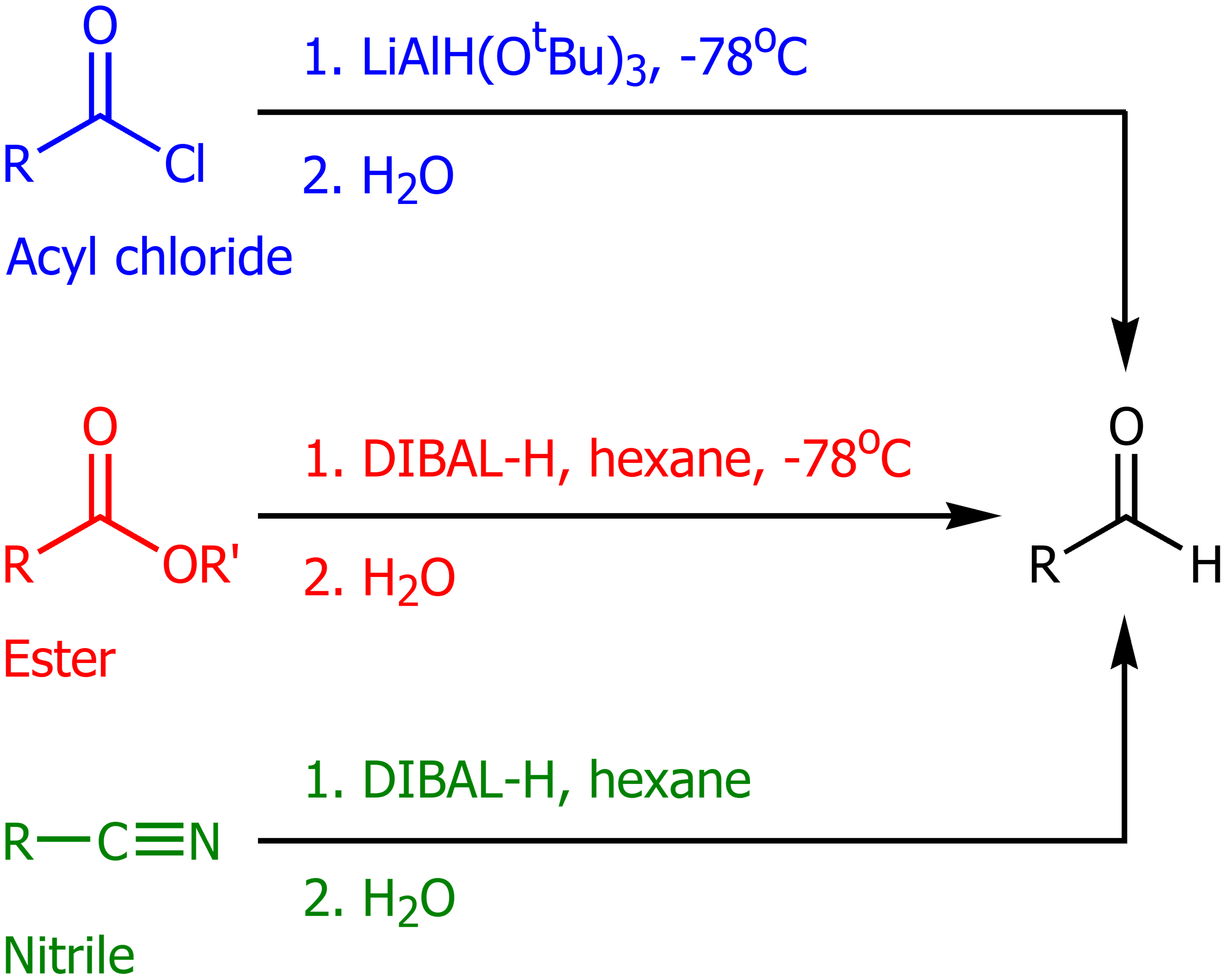
Lithium Aluminum Hydride (LAH) in the presence of an acyl chloride
LAH with (OtBu)3 in cold temps will form an aldehyde. This will also work on acylbenzenes,
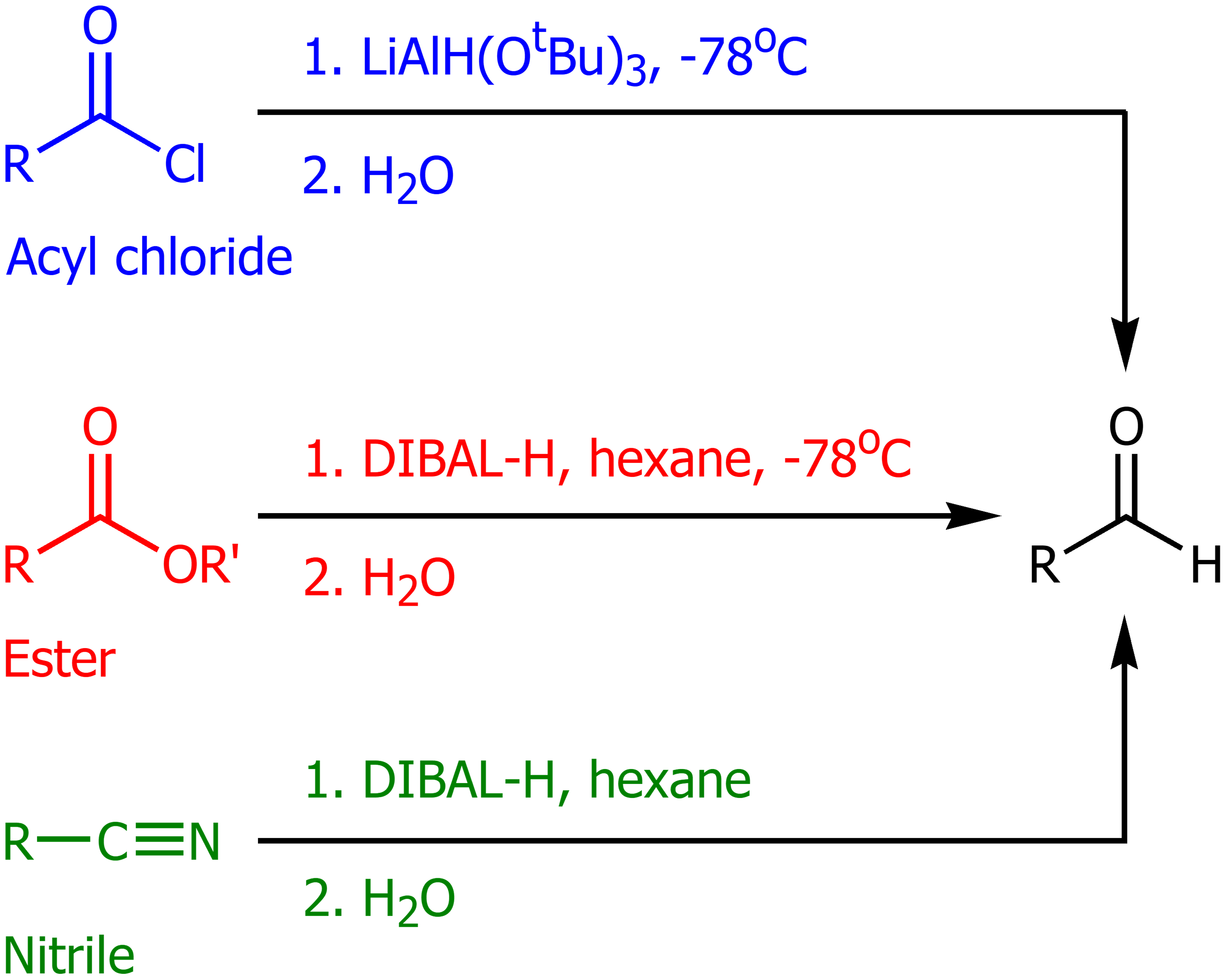
DIBAL-H in the presence of esters and nitriles
Using DIBAL-H in hexanes, then adding water will form aldehydes. In a cyclic ester this will produce an aldehyde and an alcohol.
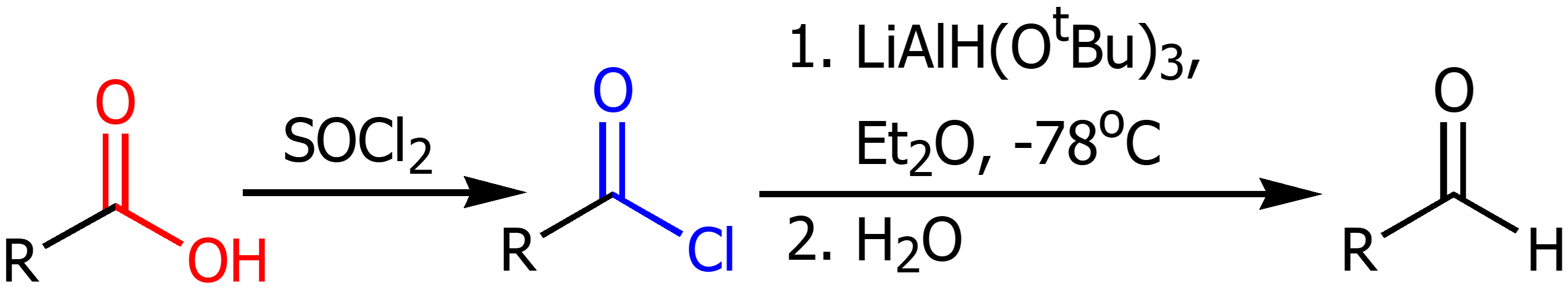
Carboxyilic Acid to Aldehyde
Using SOCl2 to form an acyl chloride then using LAH in Et2O and water to form aldehyde.
Acetals - Aldehydes
In the presence of an aldehyde using excess strong base, and strong acid will form an acetal and water. The acetal is favored at equilibrium.
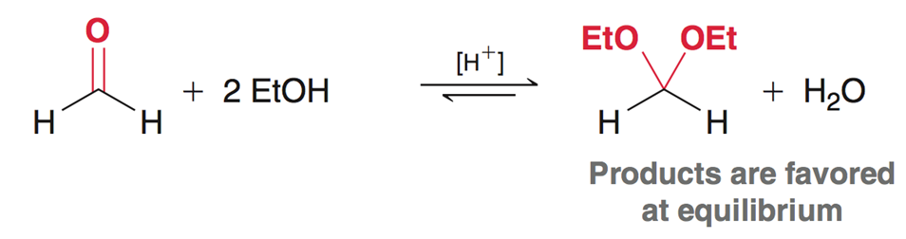
Acetals - Ketones
In the presence of a ketone using excess strong base, and strong acid will form an acetal and water. The acetal is NOT favored at equilibrium.
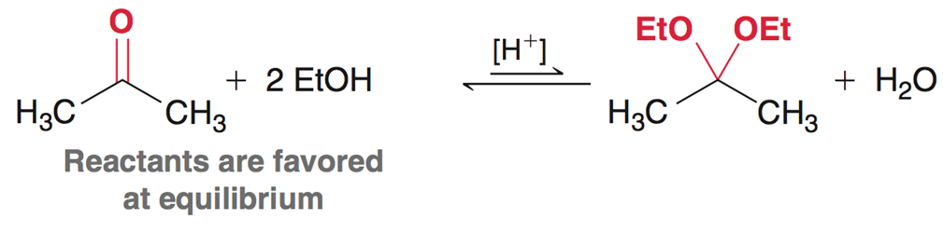
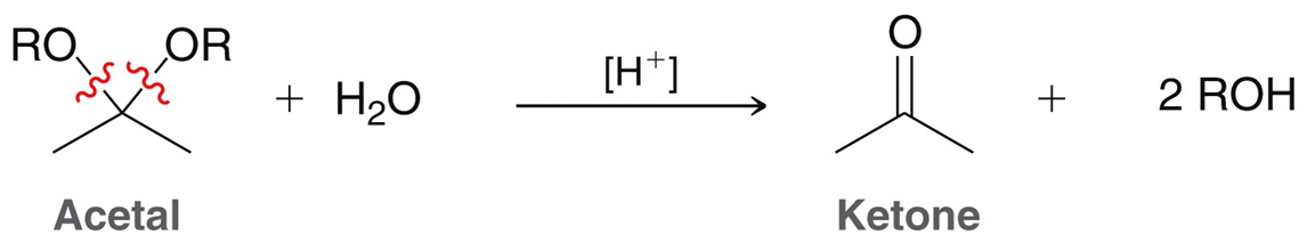
Hydrolysis of Acetals
Under acidic conditions acetals will react with water to reform the corresponding ketone/aldehyde. Acetals are stable under basic conditions and will not react.
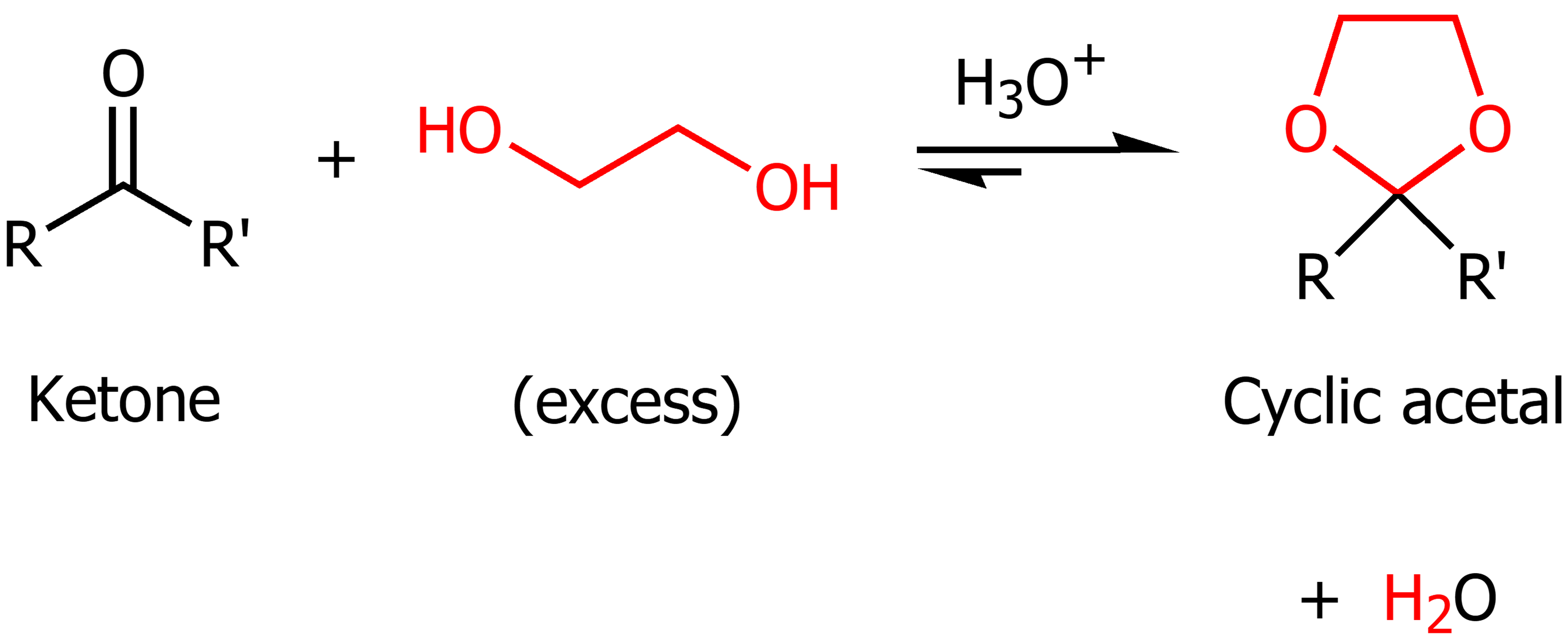
Diol in the presence of ketones
Excess diol and strong acid will turn the ketone into a cyclic acetal. This is reversible under acidic conditions.
Cylic and non-cyclic thioacetals
Ketones/aldehydes in the presence of sulfur nueclophiles will form thioacetals and thiols will form cyclic thioacetals.
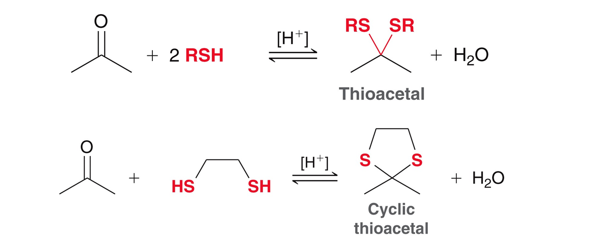
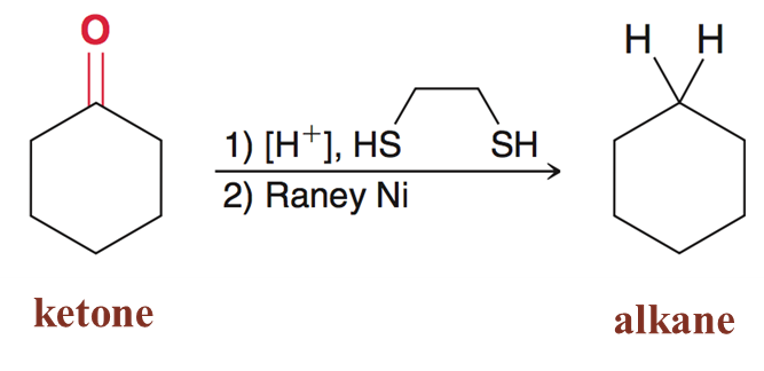
Alkanes in the presence of thioacetals
Thioacetals can be converted into alkanes when Raney nickel is present. Good for reducing ketones/aldehyde to the corresponding alkane.
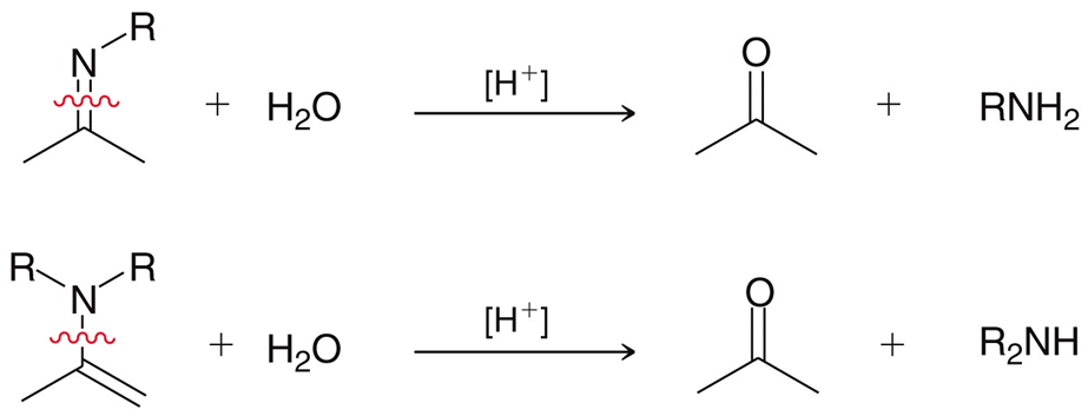
Primary amines in the presence of aldehydes/ketones
When primary amines are in the presence of a ketone/aldehyde with strong acid, it will form an imine and water. Almost identical to enamine formation.

Secondary amines in the presence of aldehydes/ketones
When secondary amines are in the presence of aldehydes/ketones with catalytic acid it will form an enamine and water. Almost identical to imine formation.

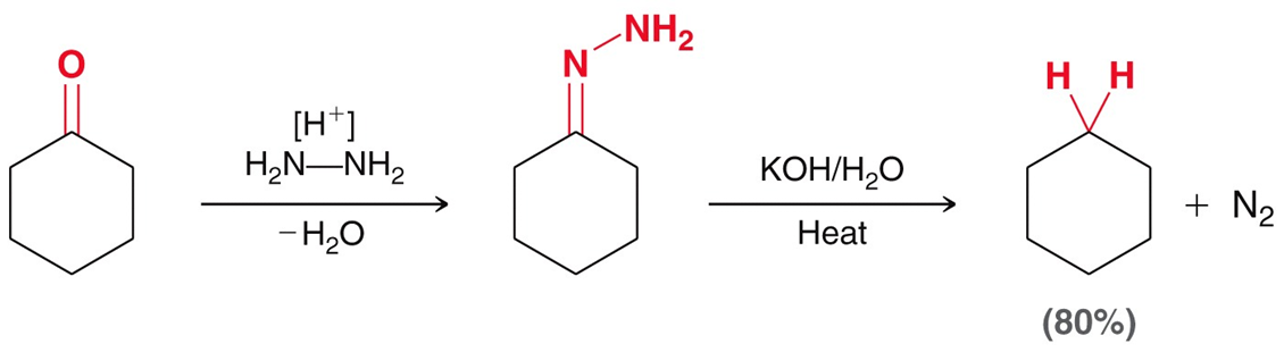
Wolff-Kishner Reduction
A two-step synthesis converting a ketone to an alkane. Using H2N—NH2 (strong acid) and dehydration. Then using a strong base/ water and heat. Similar to Clemmensen Reduction.
Hydrolysis of Imines and Enamines
Using water and strong acid, imines and enamines will oxidize back to a ketone and the respective electrophile.
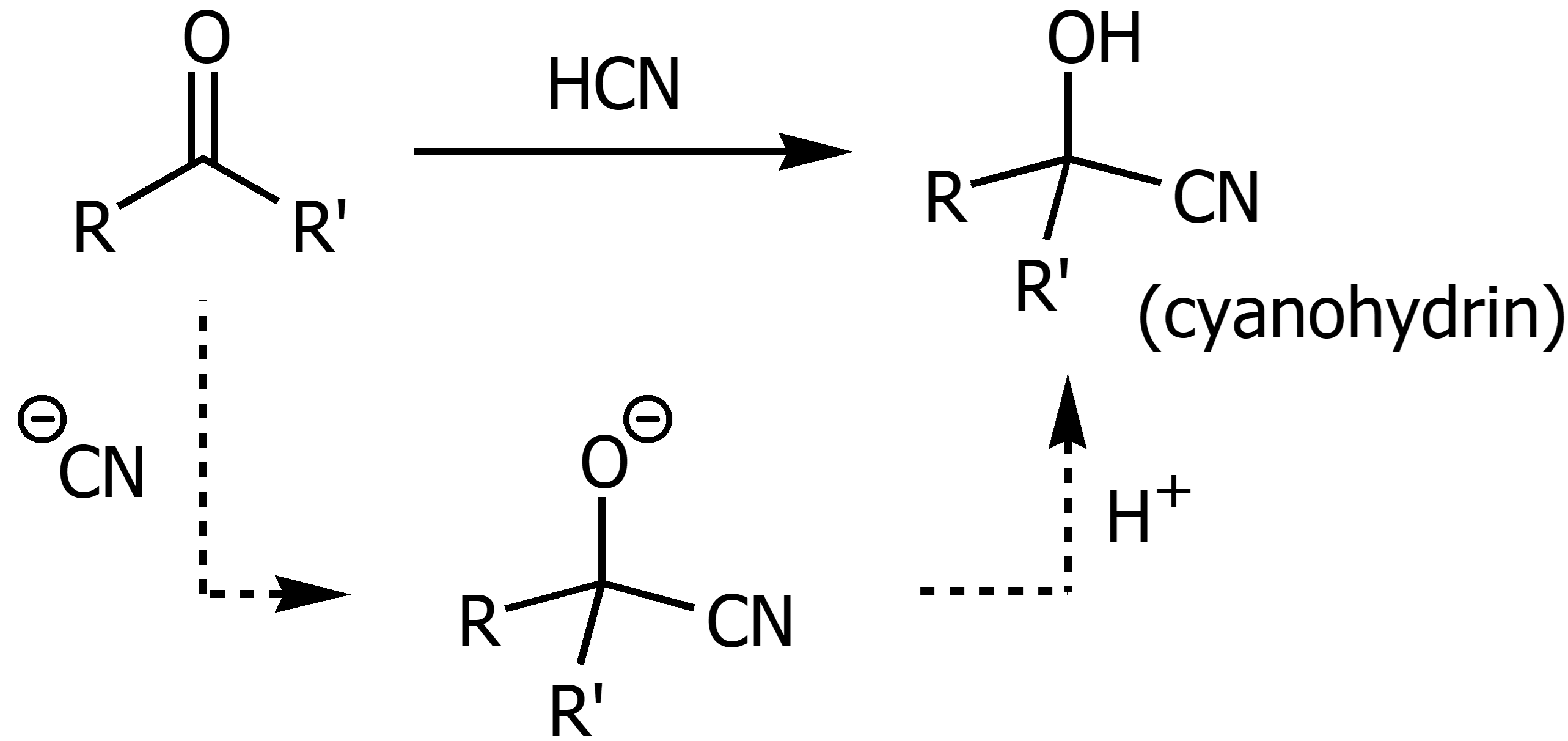
Cyanohydric Acid in the presence of ketones/aldehydes
Using HCN or cyanohydric acid on a ketone or aldehyde will result in cyanohydrin formation. This reaction works better under basic conditions so KCN is often used alongside HCN. Chirality is possible.
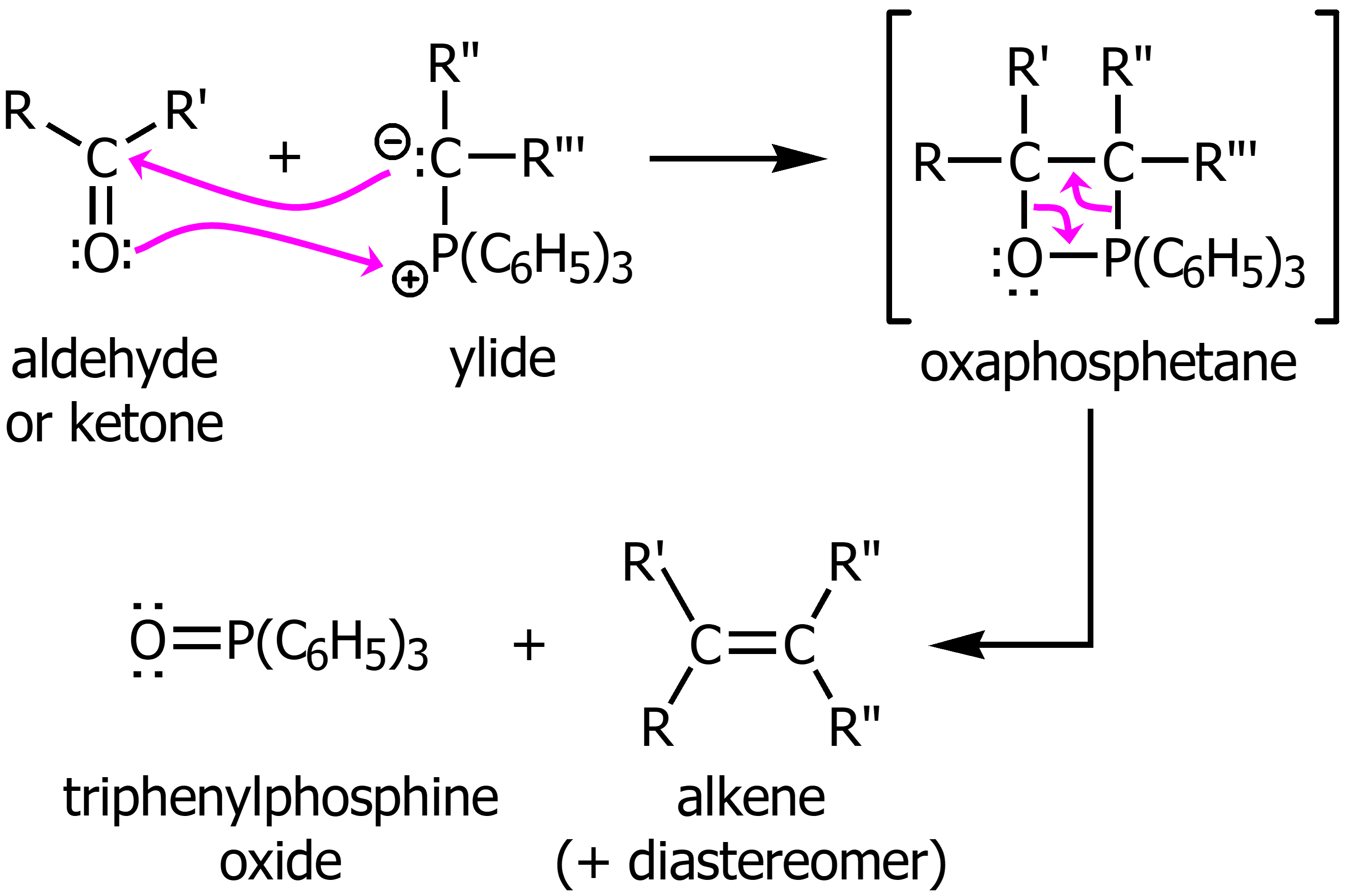
Wittg Reaction
From a ketone or aldehyde using a yilde will result in a new C=C double bond.
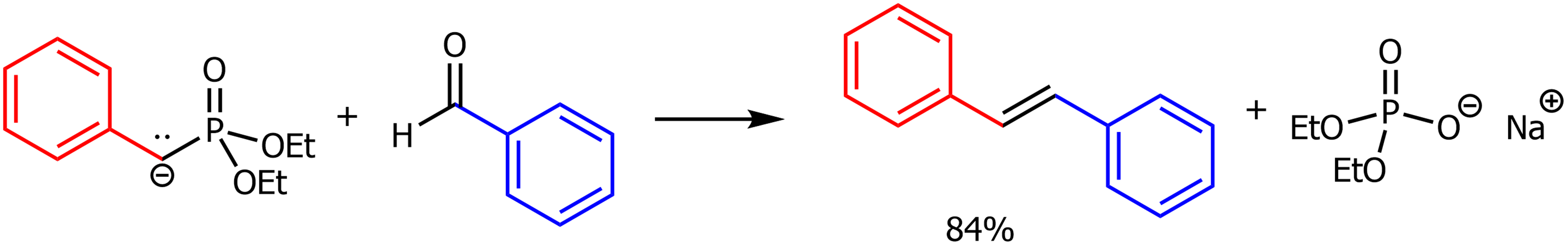
Horner-Wadsworth-Emmons (HWE) reaction
Using a reagent similar to Wittig. It is a resonance stabilitized carbanion. Made by a phosphonate ester and NaH. Used to create a C=C double bond from a ketone or aldehyde.
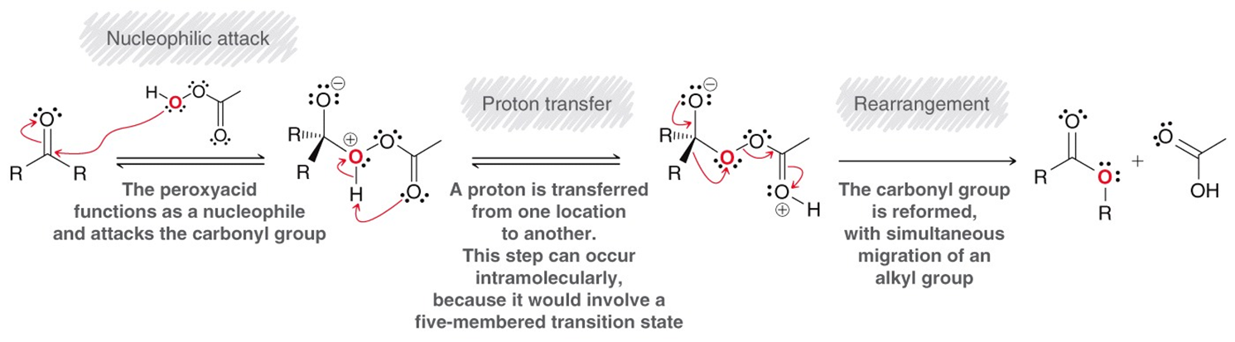
Baeyer-Villiger Oxidation
An aldehyde or ketone is converted to a carboxyilic acid acid or ester respectively. Using a peroxy acid R-COOOH / MCPBA meta-Chloroperoxybenzoic acid
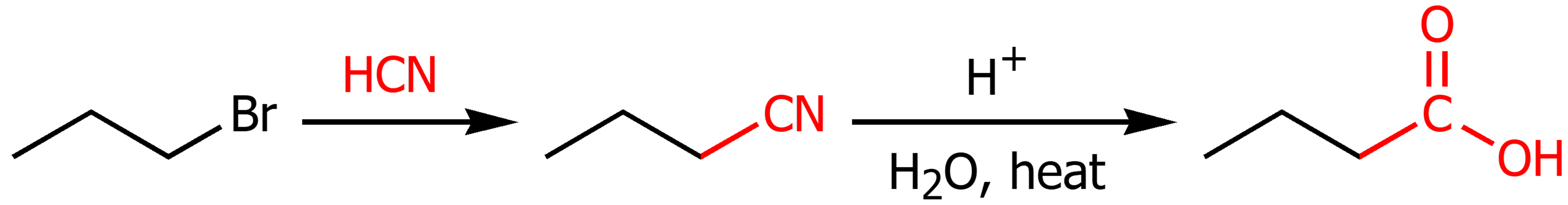
Primary Alkyl Halide to Carboxyilic acid
Using 1. HCN 2. H+ with water and heat carboxyilic acid can be synthesized.
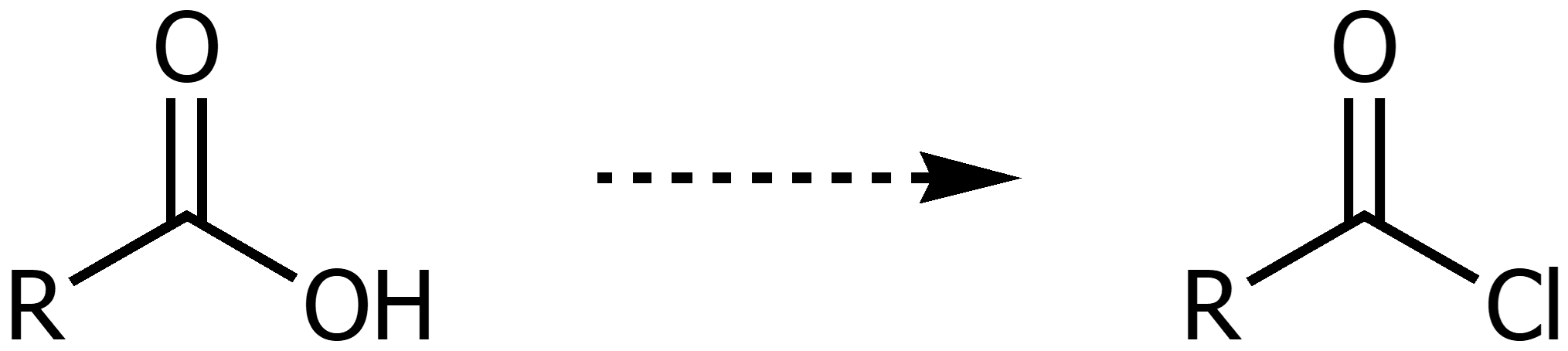
Synthesis of Acyl Chlorides from Carboxyilic Acids
Using SOCl2 (COCl)2 or PCl3/PCl5
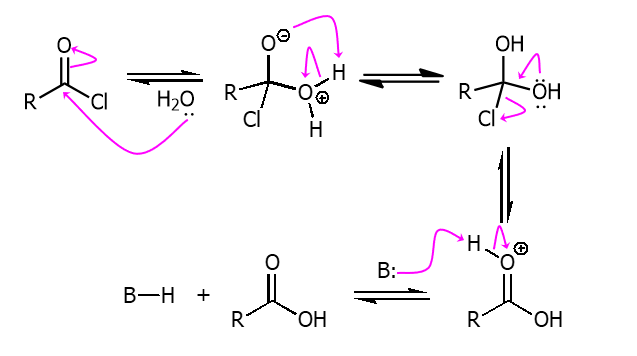
From Acyl Chloride to Carboxyilic acid
Using water and a strong base like KOH an acyl chloride can be made into a carboxyilic acid.
Acyl Chloride to Ester
Using R’OH and pyridine on an acyl chloride will produce an ester
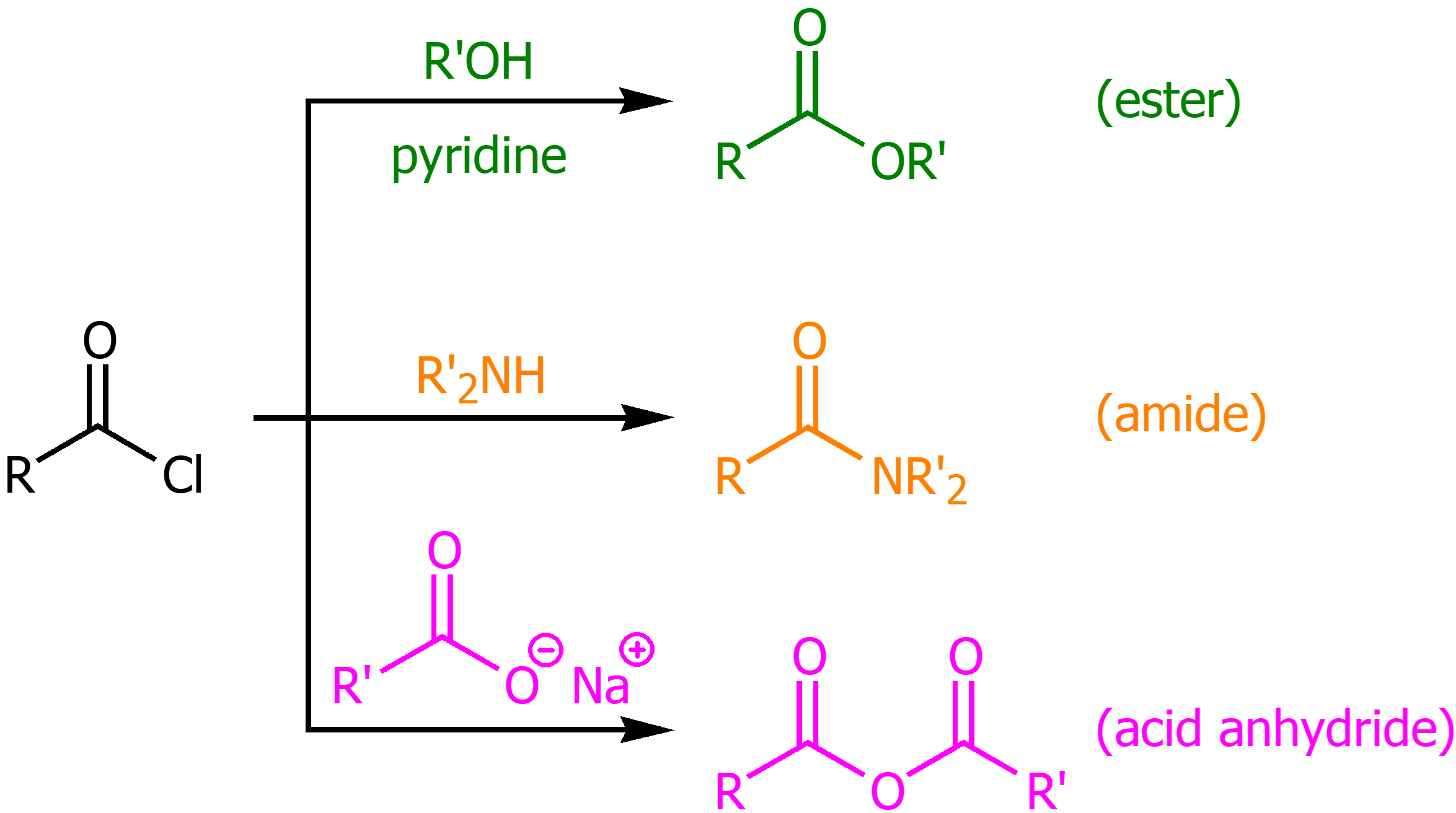
Acyl Chloride to Amide
Using R’NH2 on an acyl chloride will synthesize an ester

Acyl Chloride to an Acid Anhydride
Using a RCOOH base on an acyl chloride will synthesize an acid anhydride
Fischer Esterification
From a carboxyilic acid using MeOH (methanol) and strong acid creates an equilibria to an ester and water. The reverse of this would be hydrolysis under acidic conditions and will use the ester to produce carb. acid and methanol.
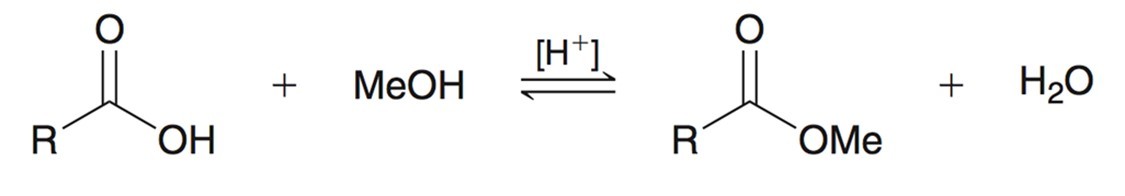
Saponification
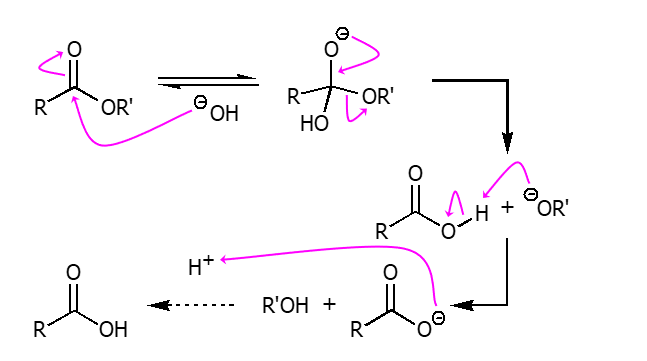
The hydrolysis of esters under basic conditions. an acid work-up is necessary to obtain the neutral carboxylic acid product. From an ester using a strong base such as NaOH then using H3O+ will synthesize the corresponding carboxylic acid and alcohol.
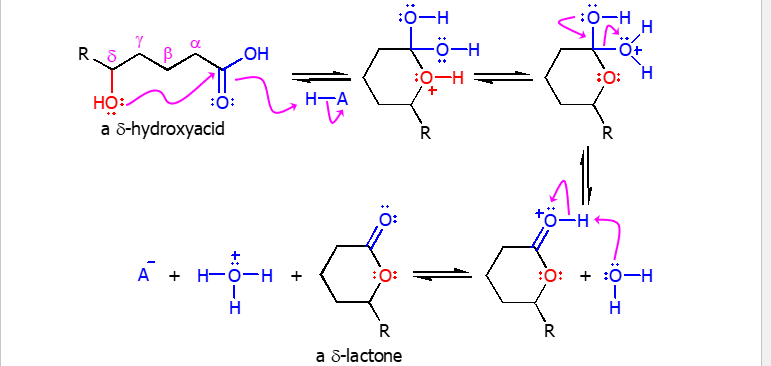
Lactone
When an alcohol and carb. acid are on the same molecule they can intramolecularly react to form a lactone.
Amides from Carb. Anhydrides
Using exccess secondary amine on a carb. anhydride will produce a amide and a carboxylate salt.

Amides from Esters
From an ester combined with secondary amine can produce an amide with the corresponding alcohol

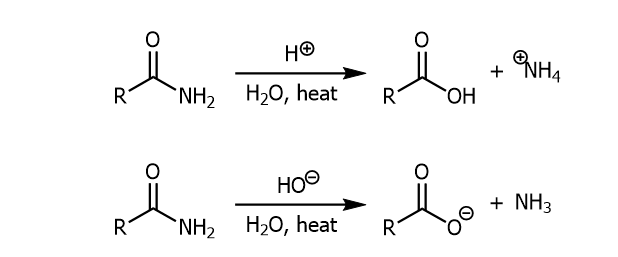
Acid/Base Hydrolysis with Amide
An amide when combined with strong acid/base with heat and water will produce a carb. acid or a carboxylate ion with ammonium or ammonia respectively.
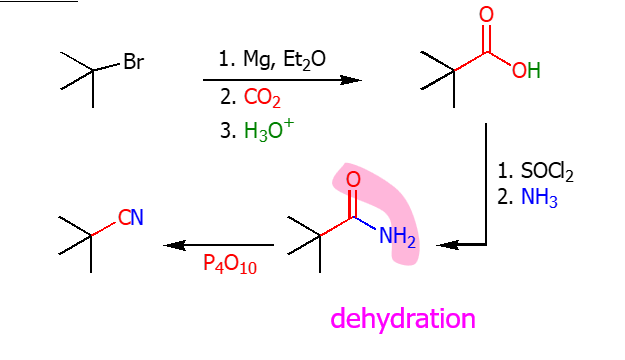
Amide into Nitrile
Using P4O10 you can dehyrdate the amide and turn it into a nitrile. This photo also shows the chemical pathway from a alkyl halide to a nitrile.
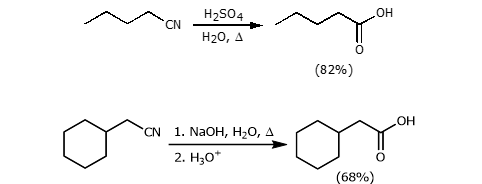
Hydrolysis of a nitrile
Adding a strong acid or base with water and heat.
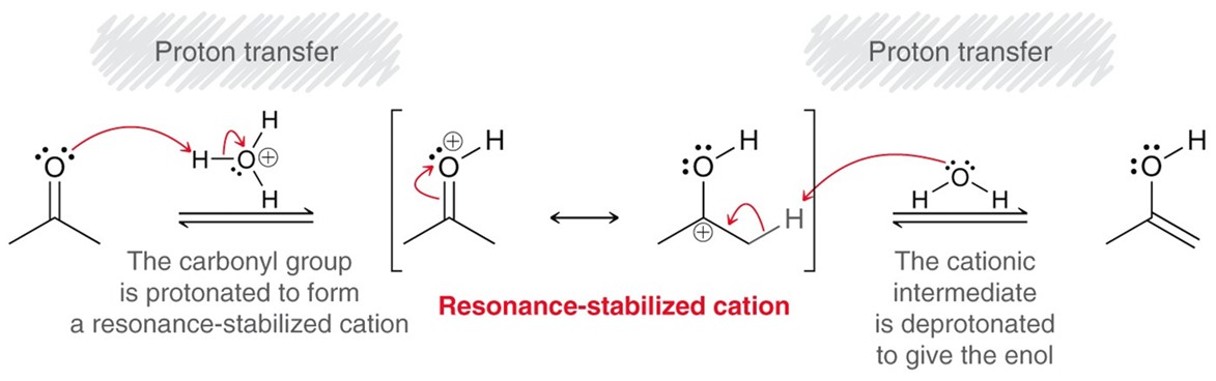
Acidic Tautomerization
By adding a strong acid to a ketone it tautomerizes into an enol
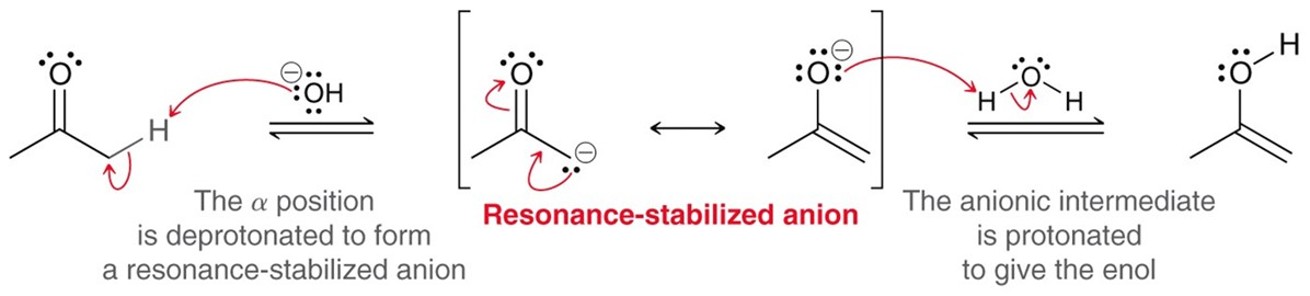
Basic Tautomerization
By adding a strong base a ketone will tautomerize into an enol
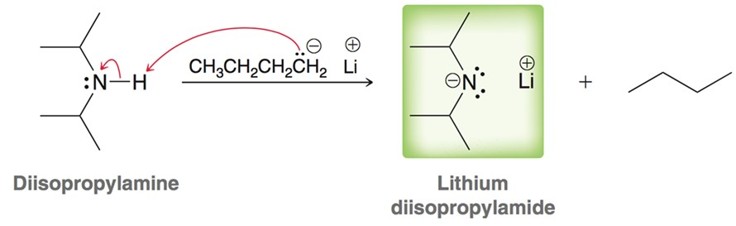
Lithium diisopropylamide for the formation of Enolates
LDA is a strong base but a very weak nucleophile and is used to remove the less hindered alpha hydrogen to form an enolate. if an aldehyde is added to an enolate a directed aldol addtion occurs.
Alpha-Halogenation Acidic conditions
Using a strong acid ketones/aldehydes can undergo alpha halogenation with a diatomic halogen gas

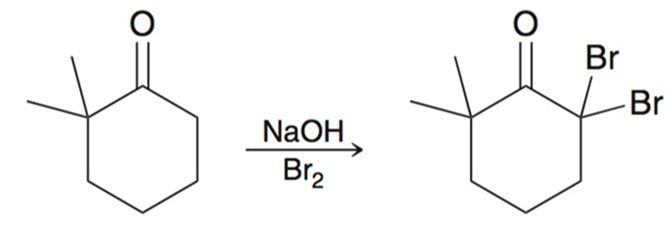
Alpha-Halogenation Basic Conditions
Under basic conditions a ketone/aldehyde will under go alpha-halogenation. This typically results in poly-halogenation and multiple of the same halogen will be added to the same carbon.
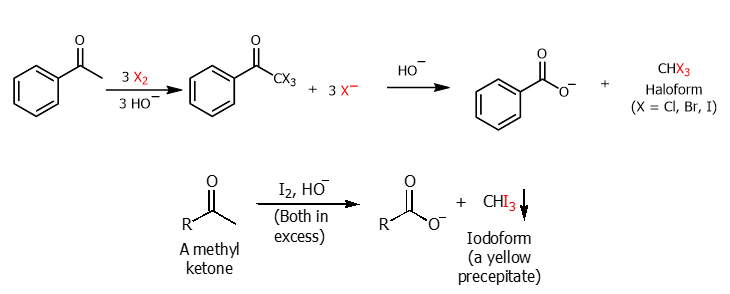
Haloform Reaction
Under basic conditions methyl ketones are converted to carb. acids using excess halogen and hydroxide
Hell-Volhard-Zelinski Reaction
Excess halogen gas and phosphorus with water in the presence of a carb acid will perform alpha halogenation
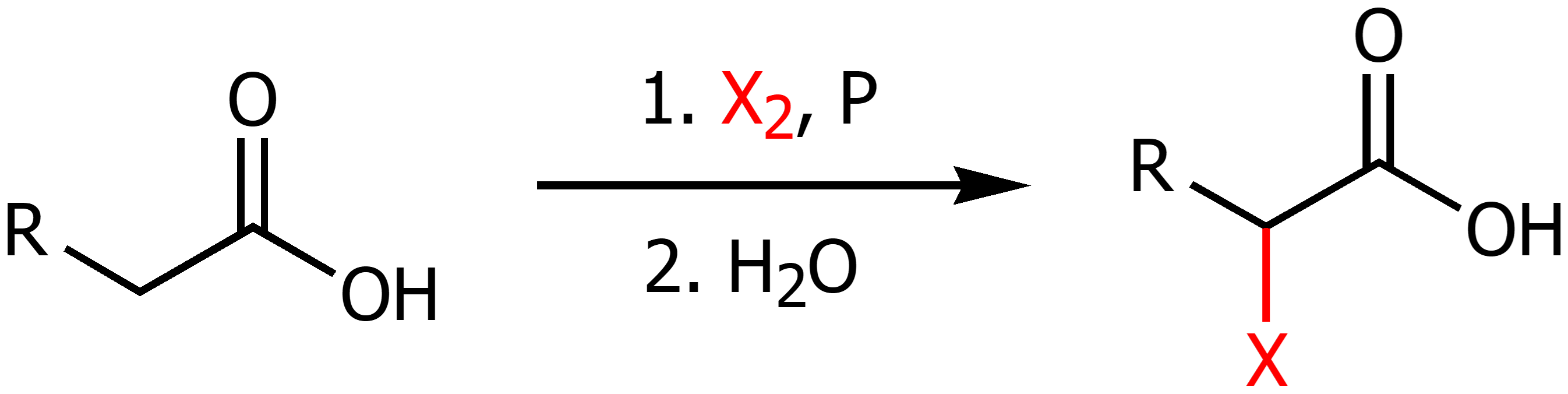
Alpha-substitution of esters
Using LDA, THF and a R-X group will substitute the alpha hydrogen for the R group.
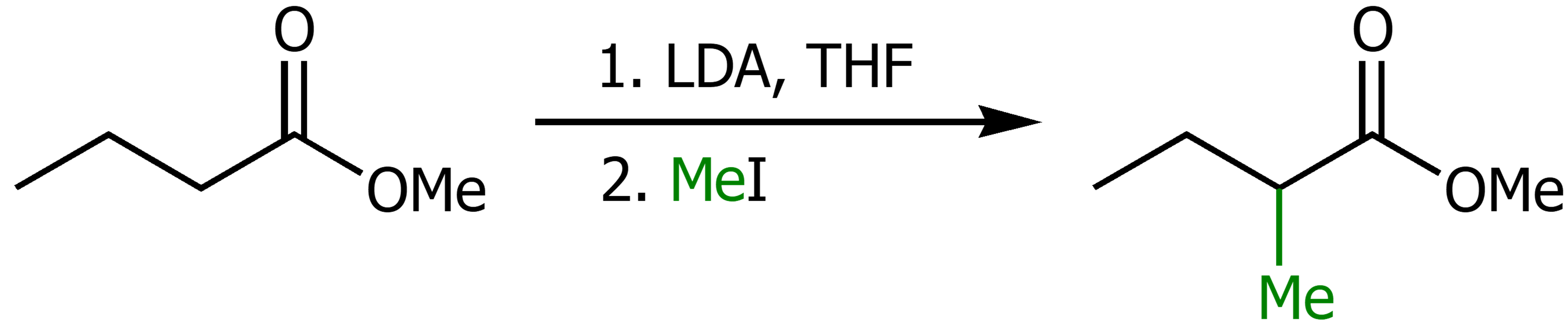
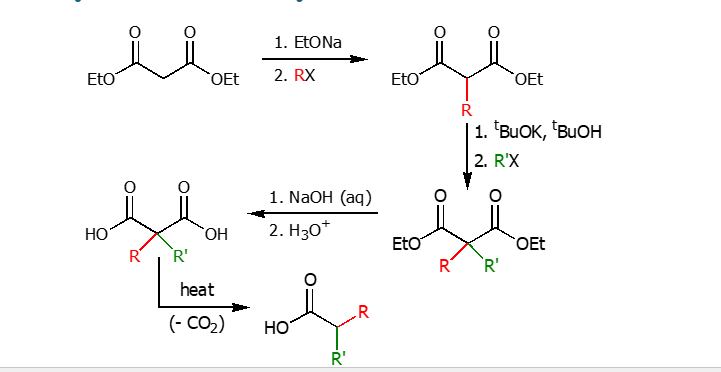
Synthesis of dialkylacetic acid
Using a strong base and a alkyl halide the alpha hydrogen is replaced with the R group. Using a tert-butyl base and another R-X group to poly akylate the middle cardbob then applying heat to separate the two ketones.
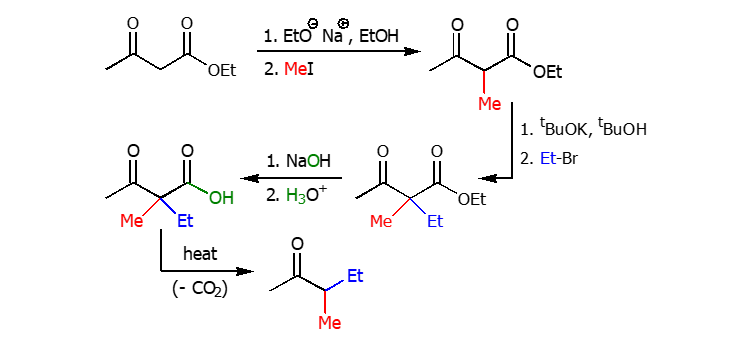
Synthesis with Acetoacetate
Similar to the Synthesis of dialkylacetic acid, the synthesis with acetoacetate uses strong bases and akyl halide groups to add R groups to connected ketones.
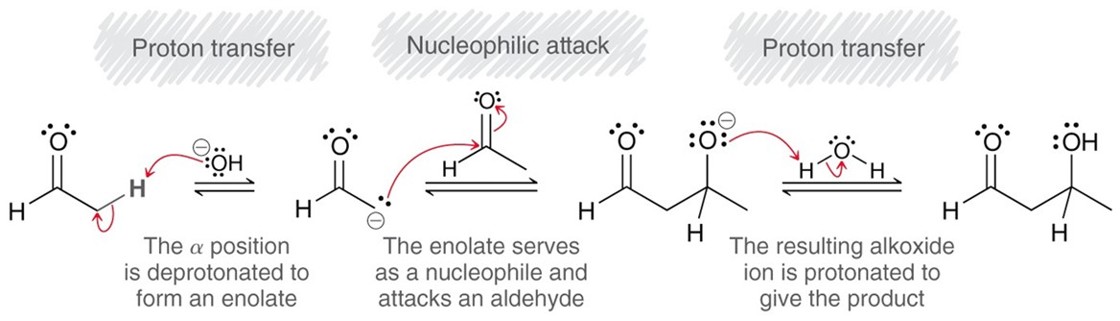
Aldol Additions
If an enolate attacks an aldehyde an aldol addition reaction occurs. Using a strong base and a water solvent. The reverse is the retro-aldol reaction.
Aldol Condensations
When an aldol is heated or placed under basic/acidic conditions an unsaturated carbonyl forms.
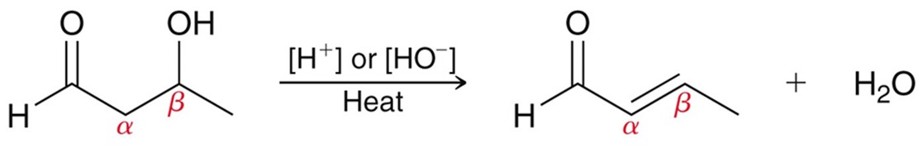
Intramoleculelar Aldol Reactions
If both an aldehyde or ketone are present on a long enough carbon chain, along with a strong base. They can undergo an intramolecular aldol reaction and form a cyclic compound with a C=C double bond.
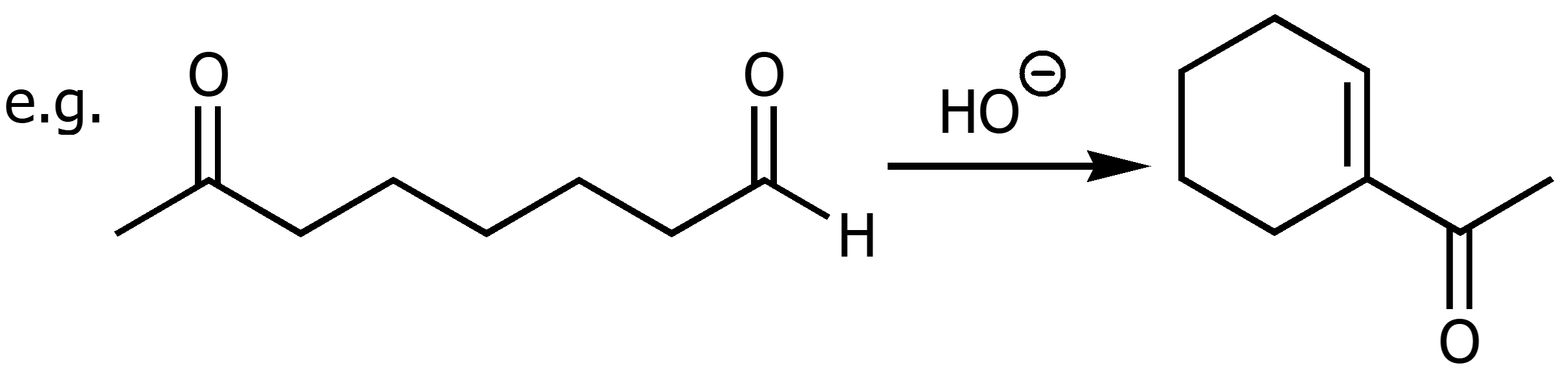
Claisen Condensations
Esters when combined witha strong base and water will form a beta-keto ester. Dieckmann cyclization are intramolecular claisen condensations.
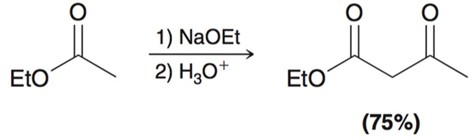
Conjugate Addition
strong nucleophiles will tend towards 1,2 whereas weaker nucelophiles will add towards 1.4
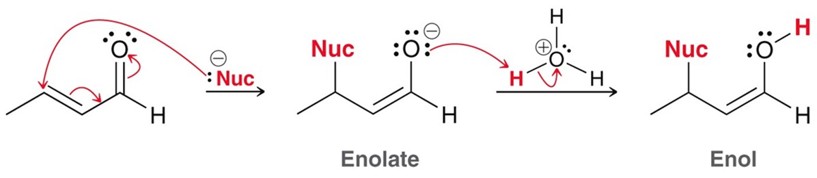
Stork-Enamine Synthesis
This takes a ketone and uses an aniline to form an enamine.

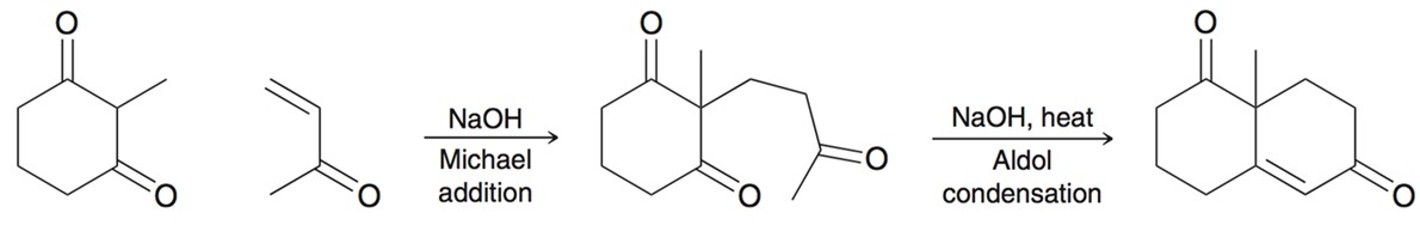
Robinson Annulation
Two steps:
Michael addition (with a strong base)
Intramolecular aldol condensation
Amine from Carboxyilic acid
SOCl2 with excess NH3 will create an amide. This can then be made into an amine using LAH and water.

Azide Synthesis
Conversion fo an akyl halide to an azide via Sn2 by a nucelophile like NaN3 then using a strong base or LAH to conver the N3 to an NH2

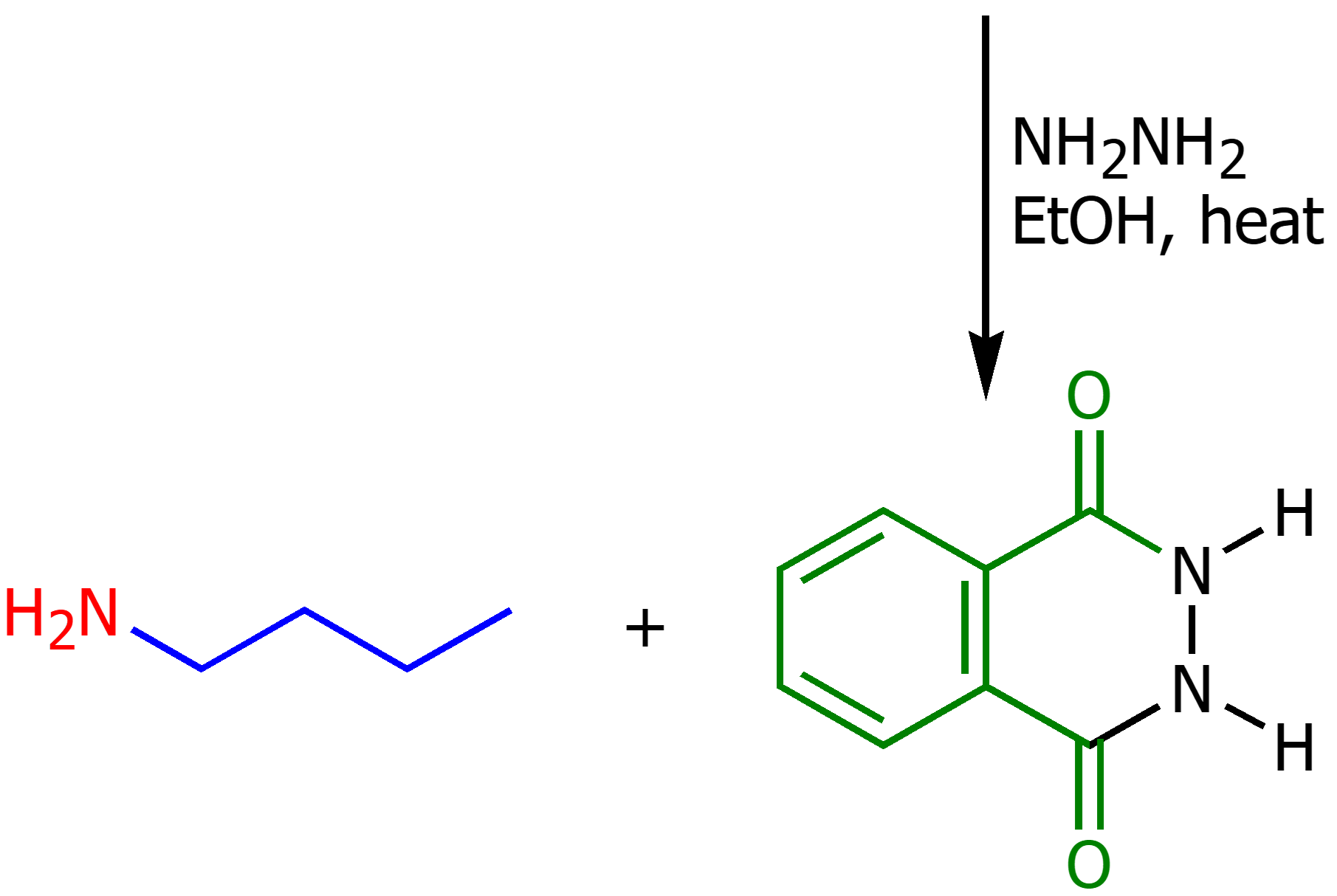
Gabriel Synthesis
Synthesis of primary and secondary amines from phthalimide. Using strong base and alkyl halide. Then using hydrazine (NH2NH2) and a strong base to produce a primary amine.
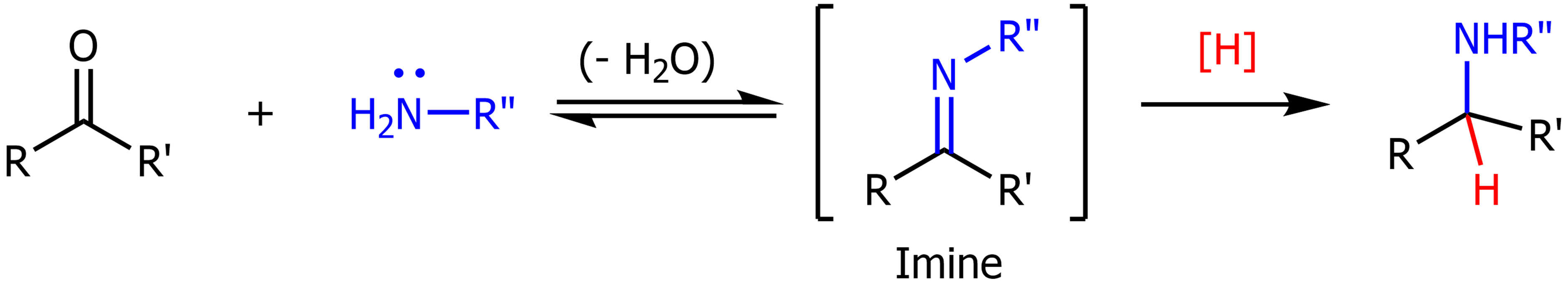
Reductive Amination
Ketone or aldehyde is converted into an imine, then into an amine using a primary amine, dehydration and water.
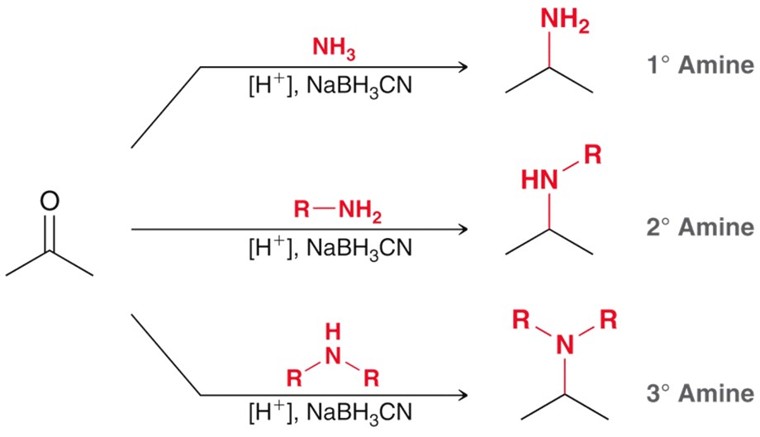
Sodium Cyanoborohydride
Similar to NaBH4. Must be formed in situ to yeild the desired amine.
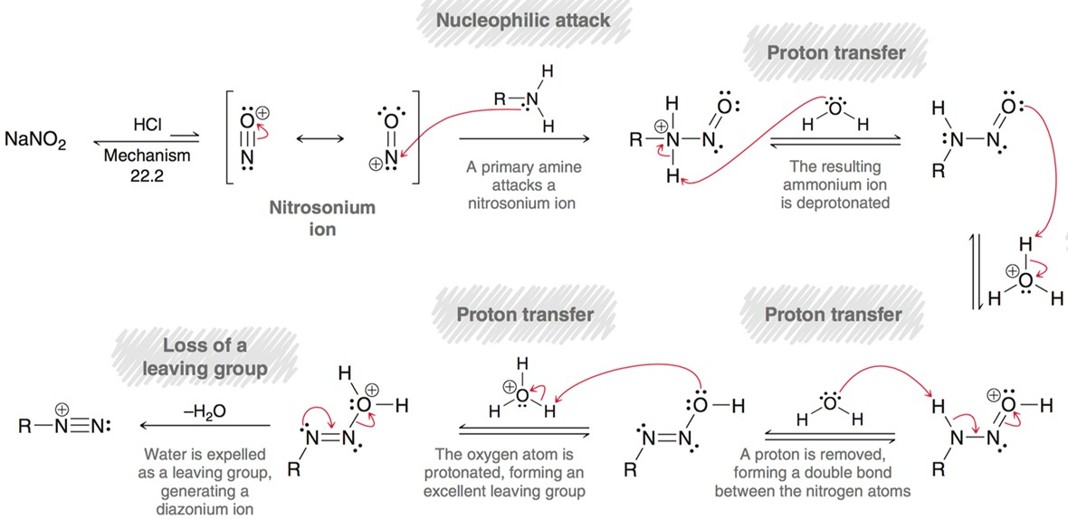
Diazotization
primary amine with NaNO2 will yield a diazonium salt. This can then be used for Sandmeyer reactions.
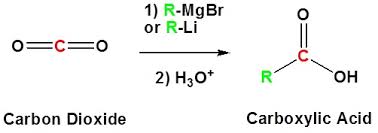
Carbon Dioxide in the presence of Grignard reagent
Using R-MgX and a strong acid. Carb. acid can be synthesized.