Chapter 13 - Bioenergenics and Biochemical Reaction Types
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
What is metabolism?
The study of synthesis and use of energy
What is the difference between catabolism and anabolism? Which one requires energy and which produces energy?
Catabolism: breaking down macromolecules into building blocks and is energy producing
Anabolism: building macromolecules from building blocks and requires energy
Where does catabolism take place?
Cytosol or mitochondria
What are the key features of metabolic pathways? (2)
1st committed step with a large -ΔG’ and makes the pathway irreversible
Catabolic and anabolic pathways are different
Do irreversible reactions have ΔG’s closer to or further from equilibrium?
Further from
What is an exergonic reaction? What is an endergonic reaction?
Exergonic: ΔG < 0 spontaneous, forward reaction favored
Endergonic: ΔG > 0 nonspontaneous, reverse reaction favored
What is dynamic equilibrium?
The rate of the forward reaction equals the rate of the reverse
What is steady state?
Input of reactants = removal of products
Observe no net change
What is flux?
The direction of flow through metabolic pathways, also known as flow
In what ways can metabolic flux be regulated? (4)
Change the concentration of substrate
Allostery control: binding of a molecule at site other than active site to induce a conformational change
Covalent modification (e.g. phosphorylation, ubiquiniation)
Making more/degrading enzyme (e.g. transcription/translation)
What is the difference between a kinase vs a phosphatase?
Kinase = +P
Phosphatase = -P
What are the high energy intermediates? (3) What are examples?
Phosphorylated intermediates: ATP
Thioester compounds: Acetyl-CoA
Reduced coenzymes: NADH, NADPH, FADH2, FMNH2
How to recognize a thioester compound
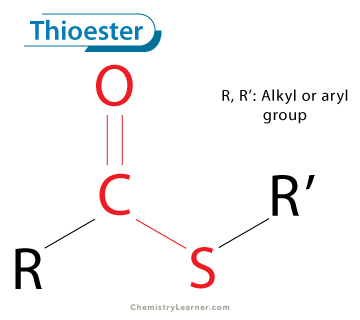
What is Acetyl-CoA?
A high energy thioester intermediate compound that contains a thioester group and an adenosine
Why is ATP a high energy compound? (4)
The hydrolysis of ATP releases energy and makes ΔG more negative.
It has resonance destabilization where electrons can move between two phosphates
It has electrostatic repulsion between O- and O- making electron density high with phosphate group
Phosphate is able to adapt stable resonance
Why are thioester compounds high energy? (2)
They have resonance stabilization with the acetyl group → acetate
S and C can pull electron density
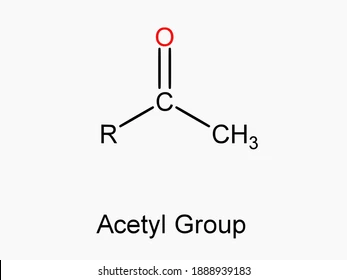
What is an acetyl group?
CH3CO-
What is CoASH?
CoA in its free thiol form (HS-)
What is a redox reaction?
Oxidation and reduction that involves the transfer of electrons
What is reduction?
A reduction of charge such as Fe3+ → Fe2+
What is oxidation?
An increase in charge such as Cu+ → Cu2+
In what ways can oxidation and reduction occur? (4)
Direct transfer of electrons
Transfer of H (1 proton/1 electron)
Gaining H = reduction
Transfer of hydride (H-)
2 electrons transferred
Adding oxygen
Losing H = oxidation
How do NAD+ and NADH differ in structure?
NAD+ is the oxidized form with the nicotinamide containing 3 double bonds.
NADH is the reduced from with the nicotinamide containing 2 double bonds.
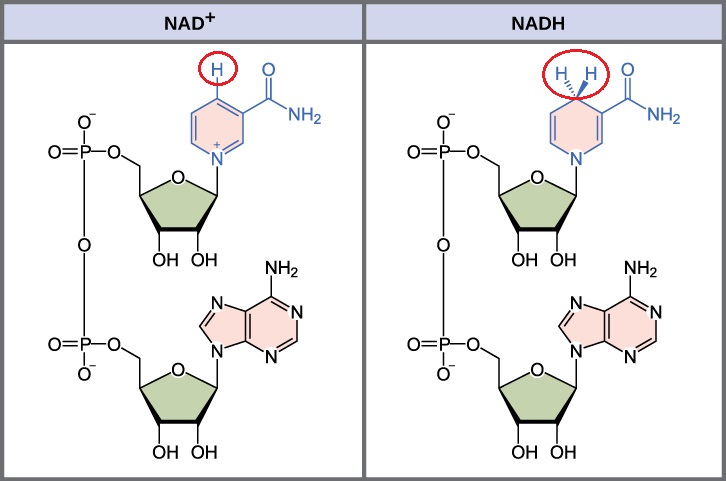
How does the reduction of NAD+ to NADH occur?
Hydride transfer
NAD+ + 2e- → NADH + H+
How do NAD+ and NADH differ in their absorbance at 340nm?
Only the reduced NADH has absorbance at 340nm
What is the difference between NAD+ and NADP+?
In NAD+, the ribose sugar has a 2’ OH
In NADPH+, the ribose sugar has a phosphate on 2’ C
How do FAD and FMN differ?
FAD: Flavin adenine dinucleotide contains isoalloxazine ring (flavin)
FMN: Missing ribose sugar ring with adenine, contains modified sugar + flavin
What is special about FAD and FMN?
they can only accept 1 H at a time, making radical intermediates until getting to their fully reduce form
What is the standard reduction potential? Why is it useful?
E = measure of affinity for electrons, energy associated with reduction.
It is important to predict whether or not redox will occur
How to determine E’?
Write the E of reduction reaction and flip the sign of E for oxidation and add them together
What is E telling you?
How easy it is to reduce a reaction
How to recognize a redox reaction
Look for elements that are uncombined (oxidation state equals 0) on one side of the equation but part of a compound (oxidation state does not equal 0) on the other side of the equation.
How to identify a more oxidized vs more reduced molecule?
More oxidized = more oxygens
More reduced = more hydrogens