AQA A level Chemistry 3.1.9 Rate equations
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is meant by the order of a reaction?
The power of concentration term in a rate equation
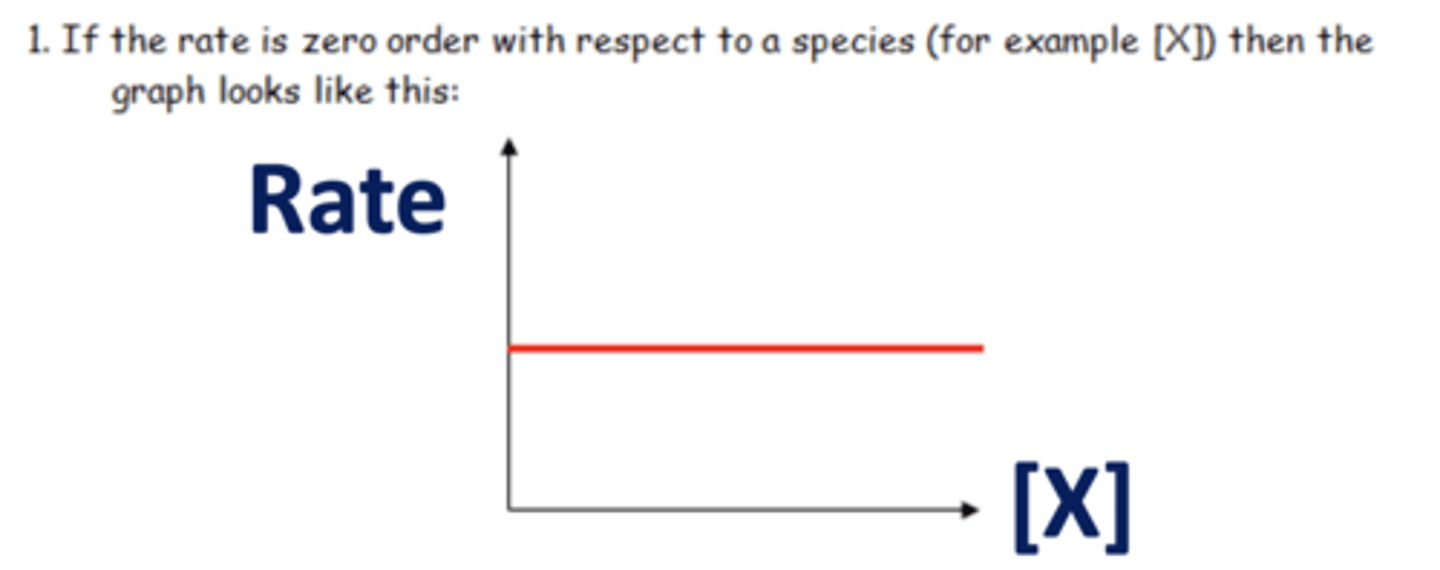
What is zero order? (1)
- As the concentration of reactants changes, the rate remains the same
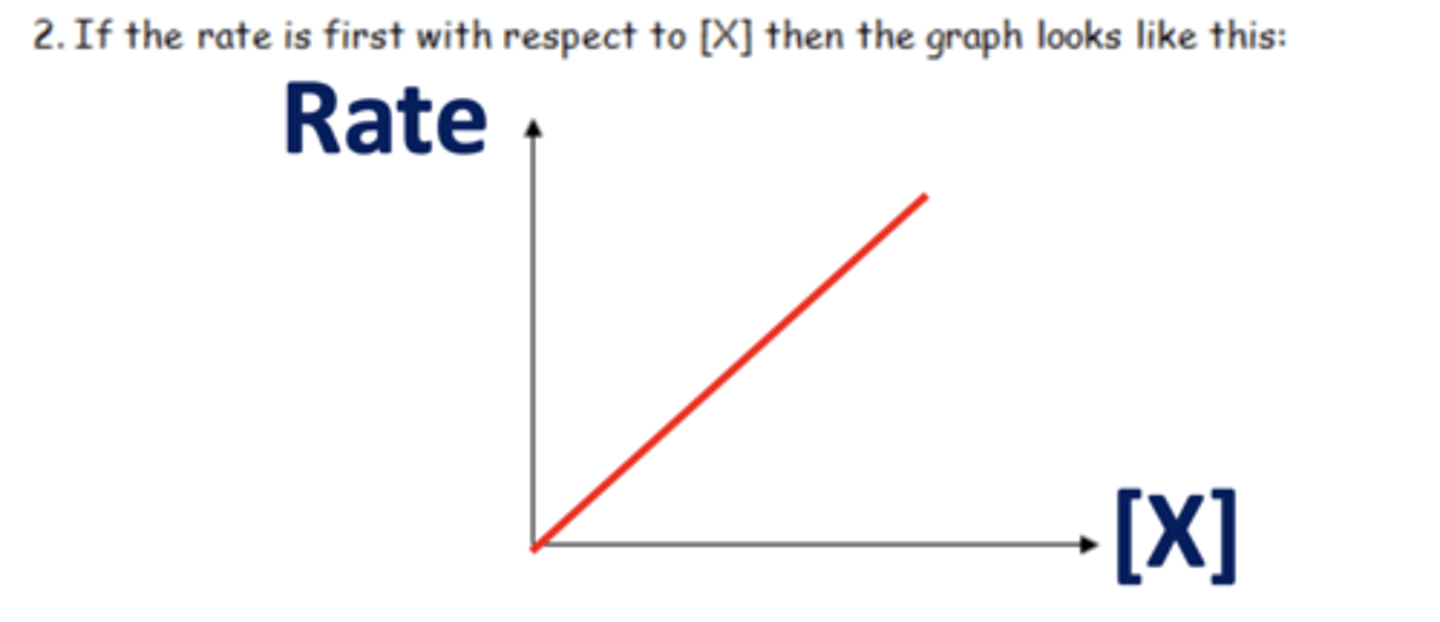
What is first order? (1)
As the concentration of reactants changes by a factor, the rate changes by the same factor
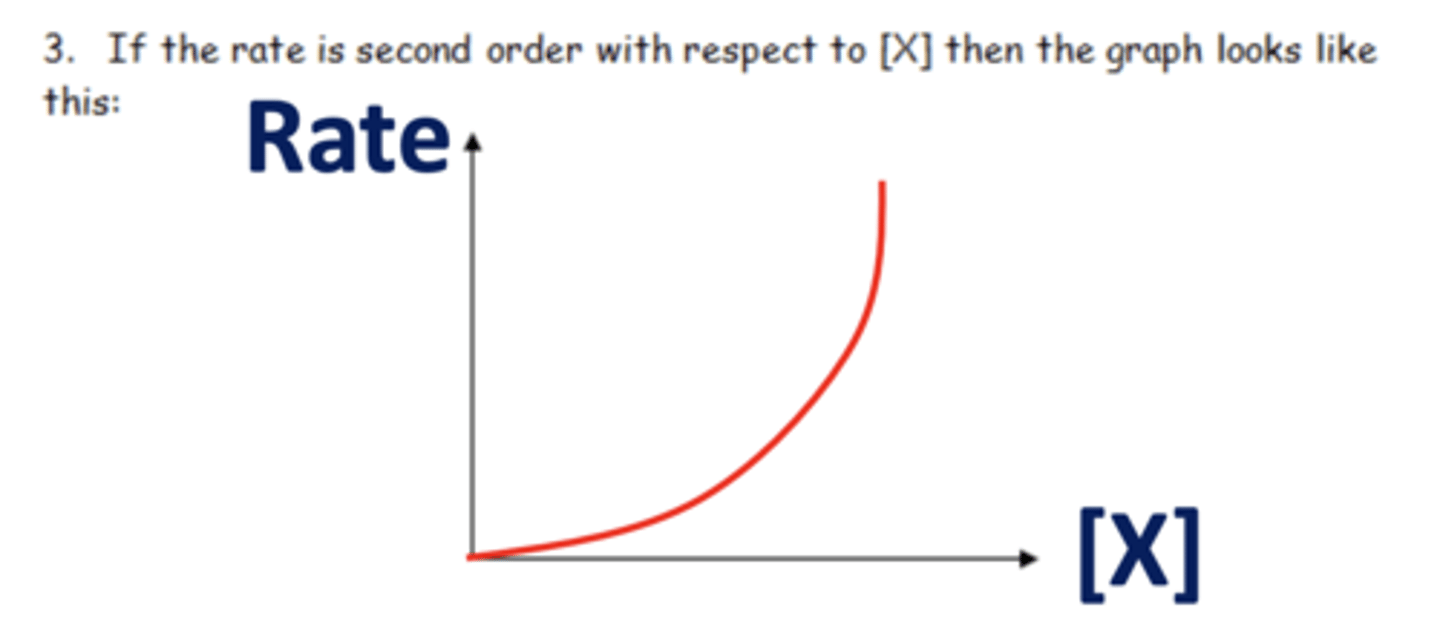
What is second order? (1)
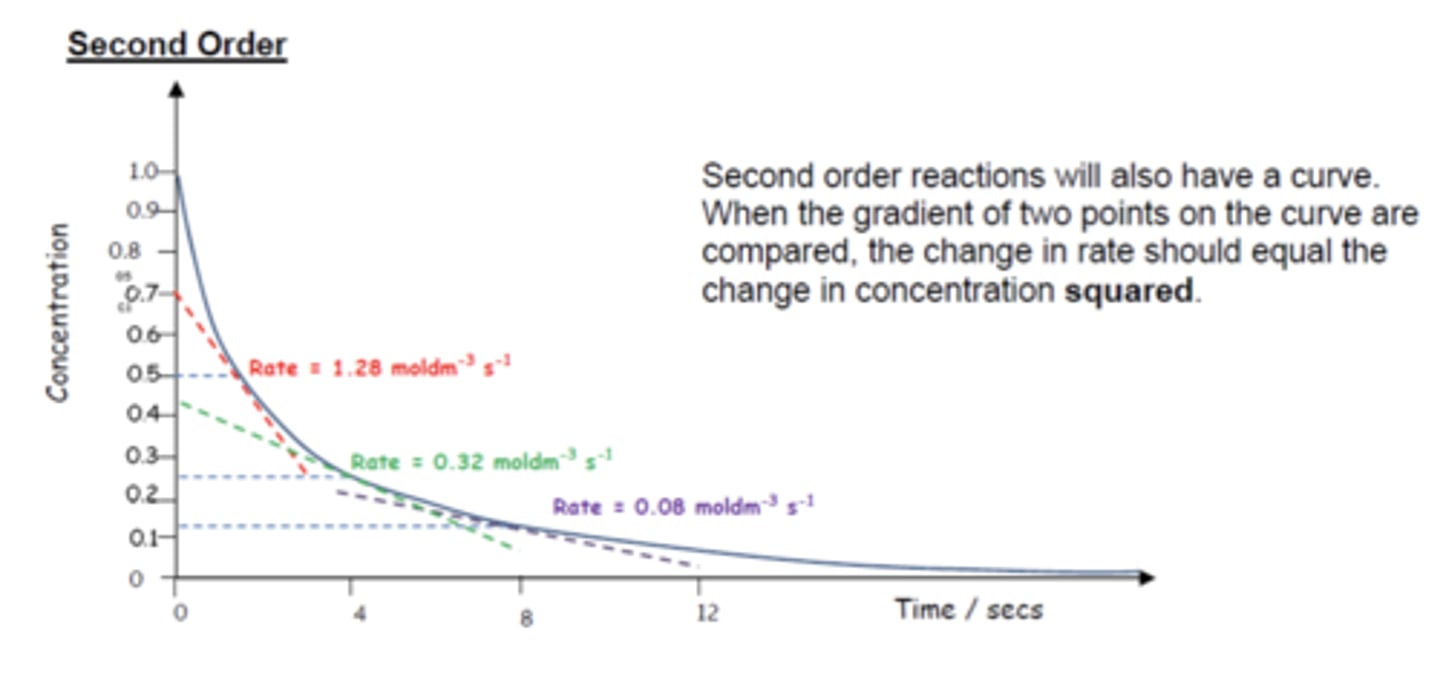
As the concentration of reactants changes by a factor, the rate increases by that factor squared
Draw a graph of zero order reaction (2)
Draw a graph of first order reaction (2)
Draw a graph of second order reaction (2)
Which reactants will not have appear in rate equation? (1)
Those that don't have an effect on the rate of reaction (zero order)
Write the general rate equation (3)

What is the overall order? (1)
Sum of all orders i.e. m+n
What is the slowest stage in a reaction mechanism called? (1)
The rate determining step
What is the relationship between the reaction mechanism and the rate equation? (1)
The steps up to and including the rate-determining step contain the same species as those shown in the rate equation, in the correct ratio.
What is the rate constant k? (1)
Relates the reactant concentrations to the rate at a particular temperature
What is the relationship between rate and k? (2)
- As rate increases, the value of k also increases
- This relationship is described by arrhenius equation
State the Arrhenius equation (4)
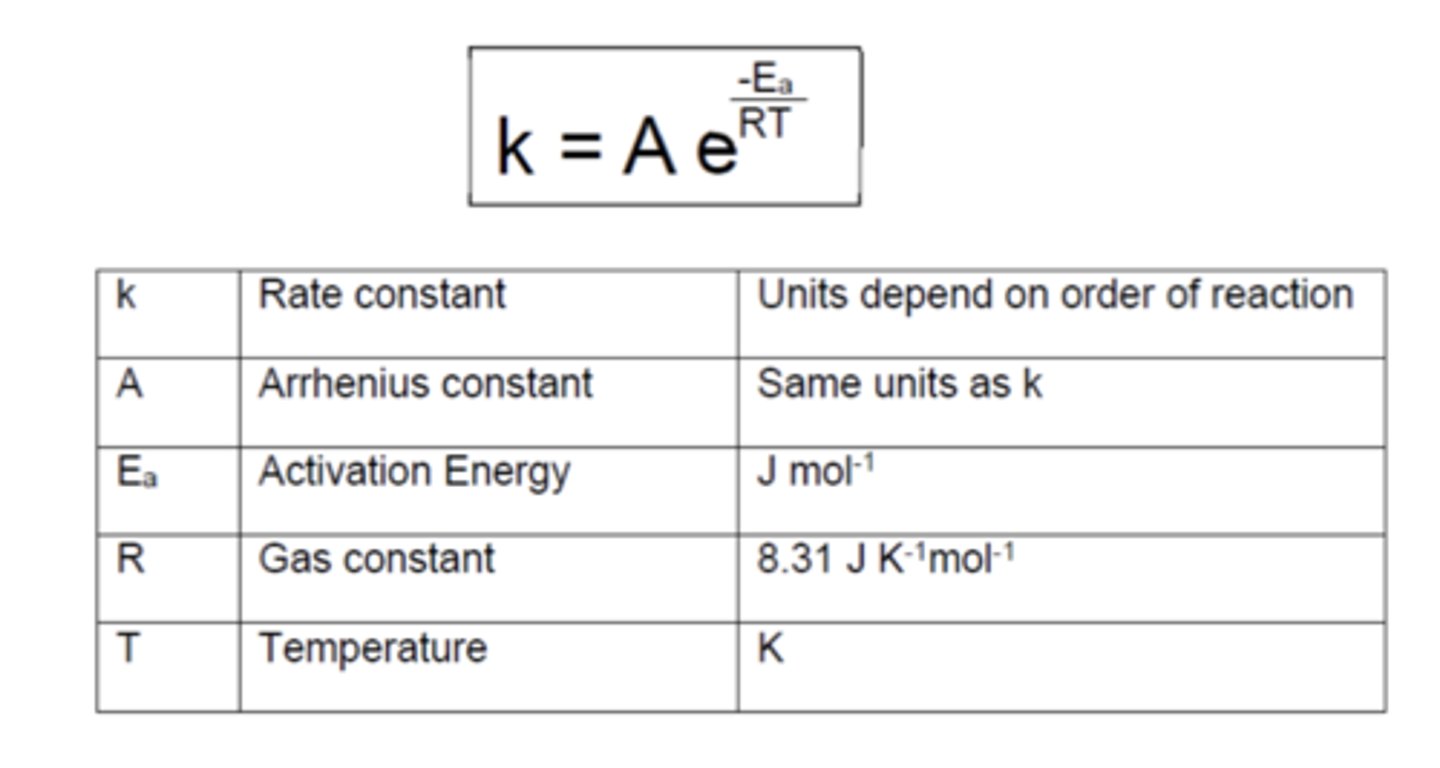
State the logarithmic Arrhenius equation (4)
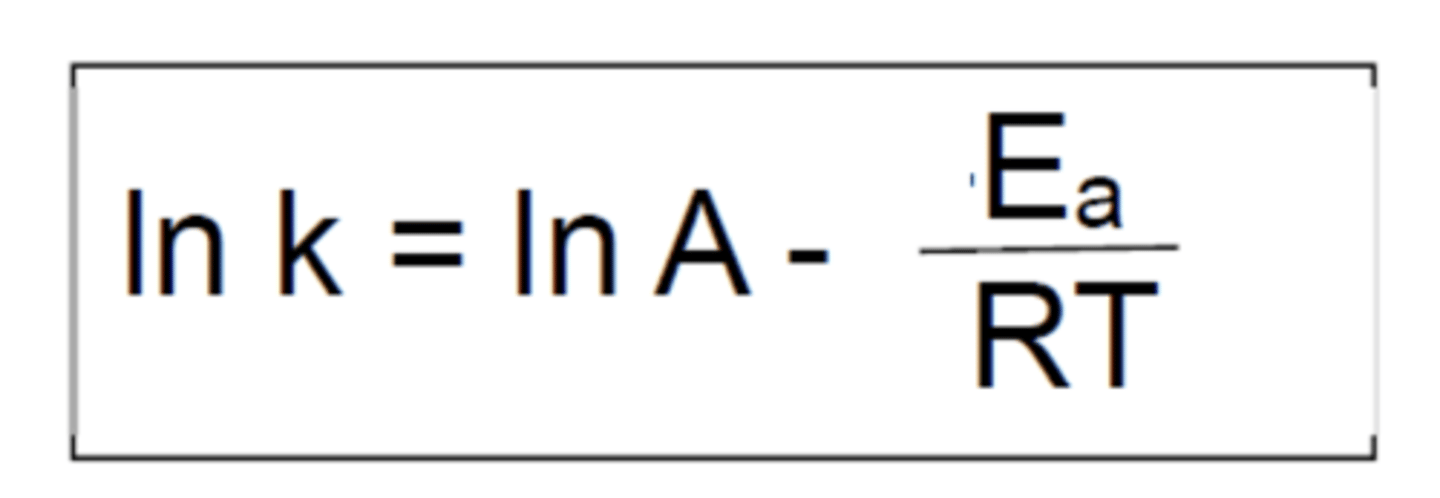
In graphical analysis of arrhenius equation, what is represented by the gradient?
-Ea / R
In graphical analysis of arrhenius equation, what is represented by the y intercept?
LnA
In graphical analysis of arrhenius equation, what is represented by y?
lnK
In graphical analysis of arrhenius equation, what is represented by x?
1/T
How does a concentration-time graph look like for zero order reactions? (3)
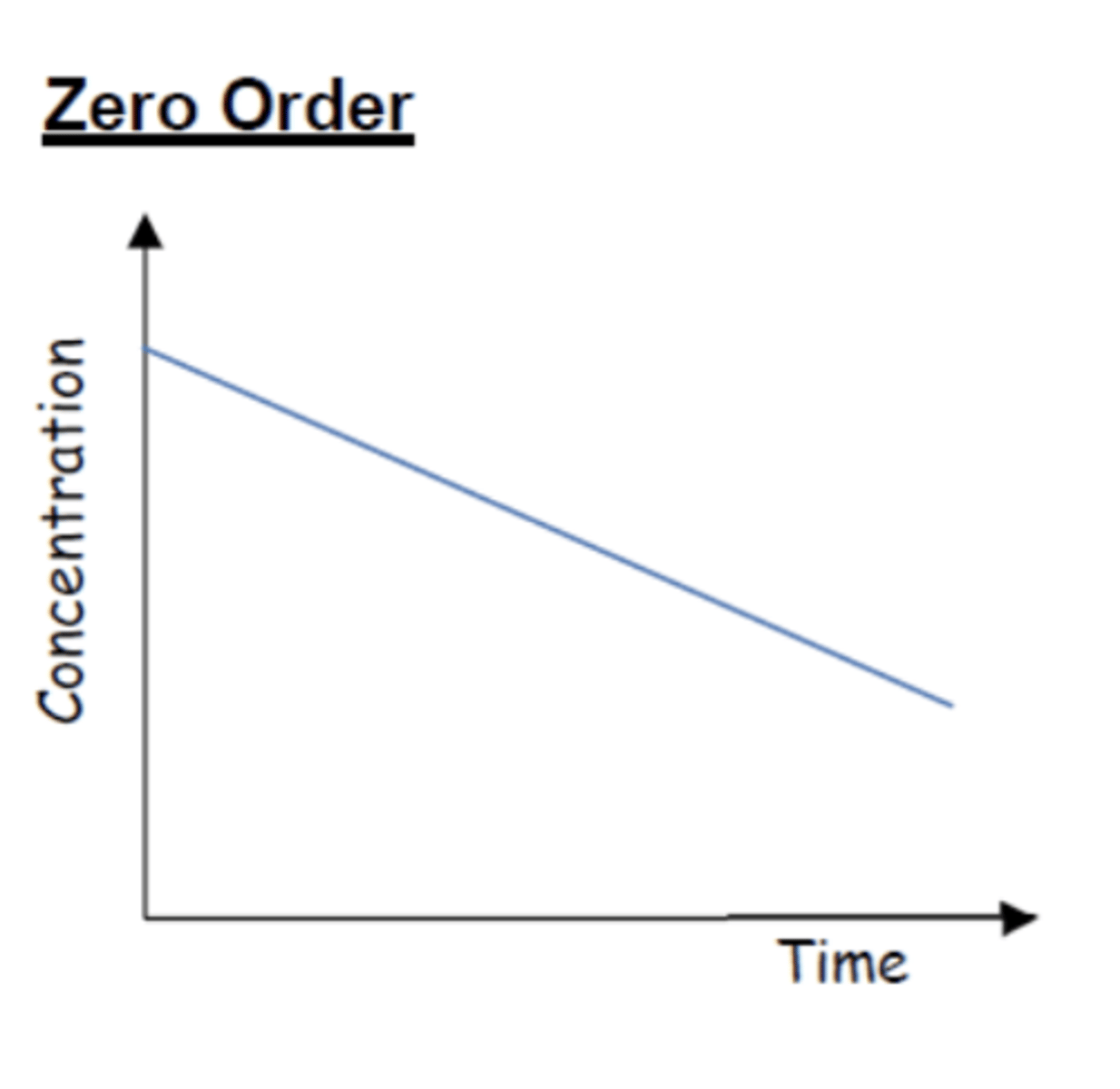
How does a concentration-time graph look like for first order reactions? (3)
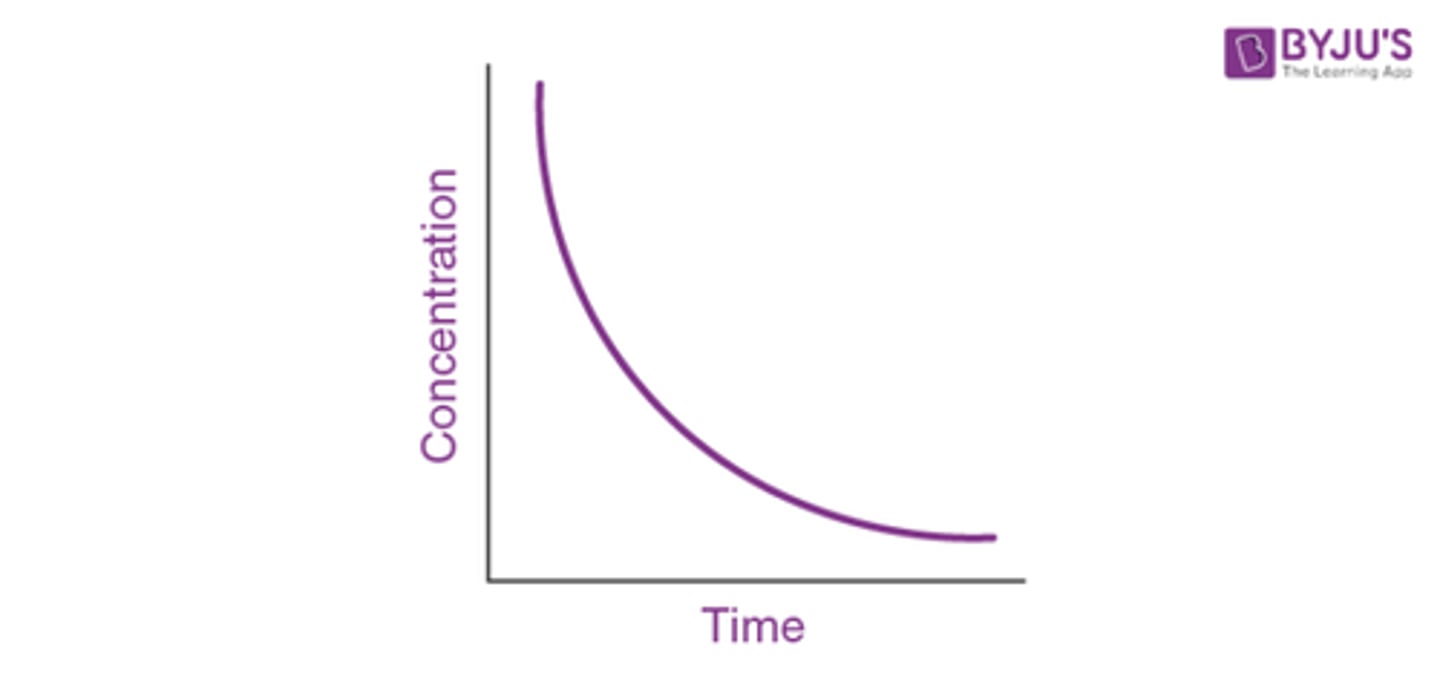
How does a concentration-time graph look like for second order reactions? (3)