9.1- 9.3: Reversible Reactions
When u heat copper (II) sulfate crystals….


so the reaction can go i either direction: it is reversible
The reaction we start with (1 above) is called forward reaction.
Reaction 2 is the back reaction.
CuSO4.5H2O (s) ⇌ CuSO4 (s) + 5H2O (l)
the reversible arrow and Chemical equation for reversible reactions :
- When writing chemical equations for reversible reactions, two arrows are used to indicate the forward and reverse reactions
- Each one is drawn with just half an arrowhead – the top one points to the right, and the bottom one points to the left: ⇌
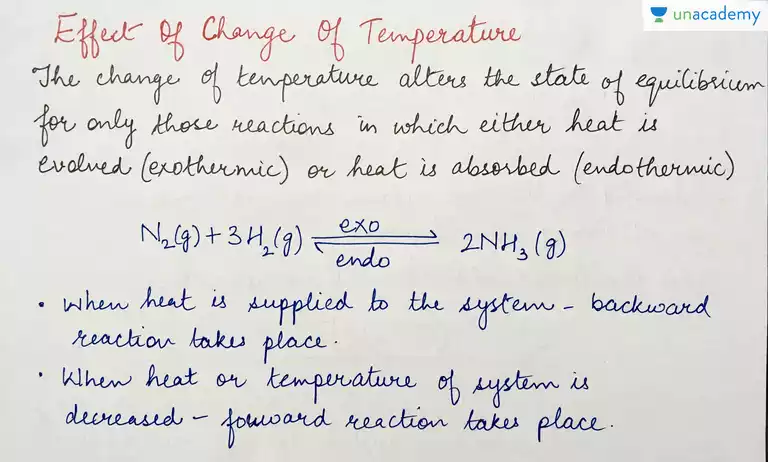
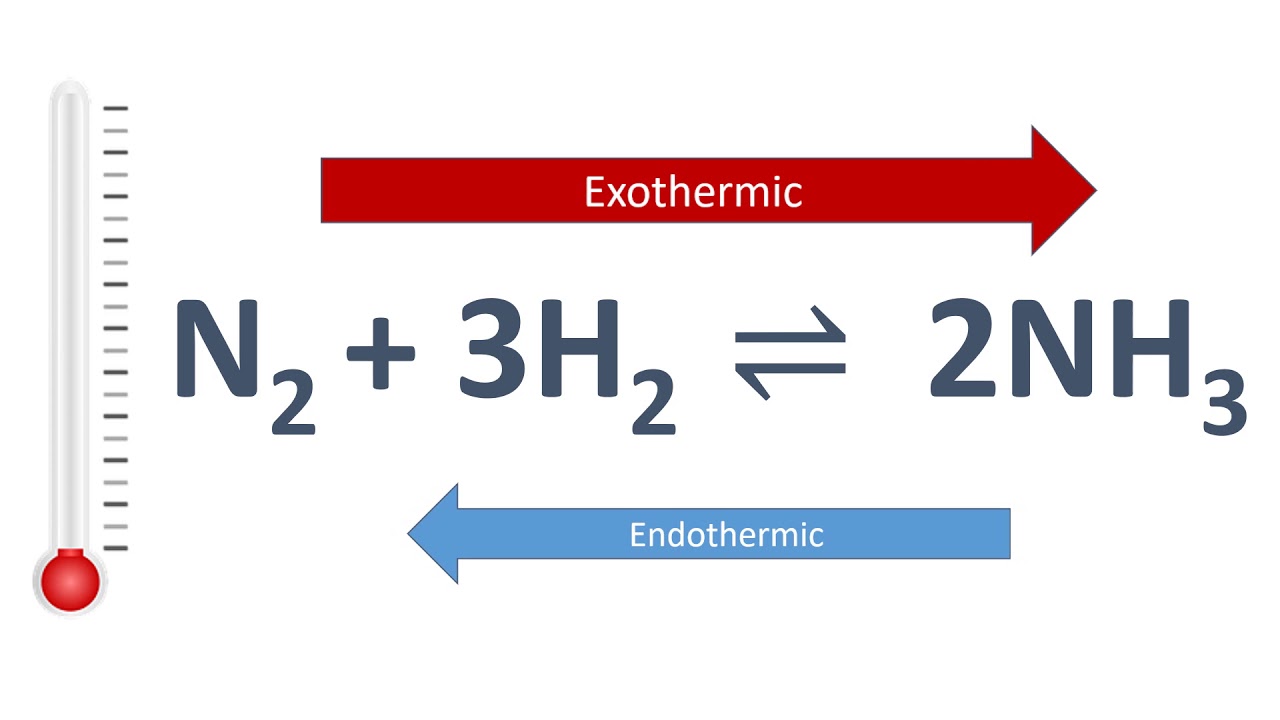
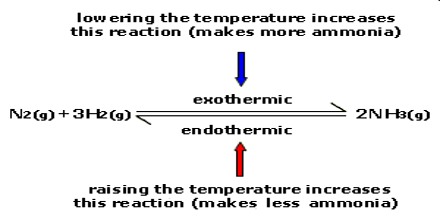
Example= The reaction for the Haber process which is the production of ammonia from hydrogen and nitrogen:
N2 + 3H2 ⇌ 2NH3
Hydrated and anhydrous salts
Let’s look closer at the example we gave of Copper (II) sulfate and explain the process in detail 😁
- Hydrated salts are salts that contain water of crystallization which affects their molecular shape and colour
- Water of crystallisation is the water that is stoichiometrically included in the structure of some salts during the crystallisation process
- A common example is copper(II) sulfate which crystallises forming the salt copper(II) sulfate pentahydrate, CuSO4**.**5H2O
- Water of crystallisation is indicated with a dot written in between the salt molecule and the surrounding water molecules
- Anhydrous salts are those that have lost their water of crystallisation, usually by heating, in which the salt becomes dehydrated
Dehydration of Hydrated Copper (II) sulfate
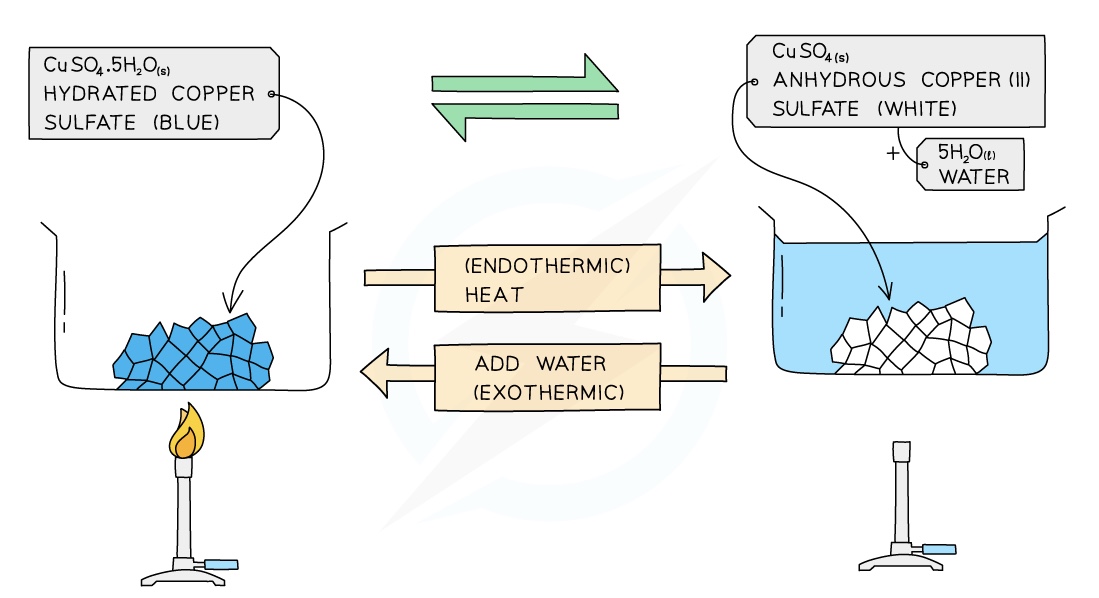
hydrated copper(II) sulfate ⇌ anhydrous copper(II) sulfate + water
Explanation
When anhydrous copper(II) sulfate crystals are added to water they turn blue and heat is given off (exothermic); this reaction is reversible
When copper(II) sulfate crystals are heated in a test tube, the blue crystals turn into a white powder and a clear, colourless liquid (water) collects at the top of the test tube
The form of copper(II) sulfate in the crystals is known as hydrated copper(II) sulfate because it contains water of crystallisation
When hydrated copper(II) sulfate is heated, it loses its water of crystallisation and turns into anhydrous copper(II) sulfate:
CuSO4.5H2O (s) ⇌ CuSO4 (s) + 5H2O (l)
Dehydration of Hydrated Cobalt (II) Chloride
hydrated cobalt(II) chloride ⇌ anhydrous cobalt(II) chloride + water
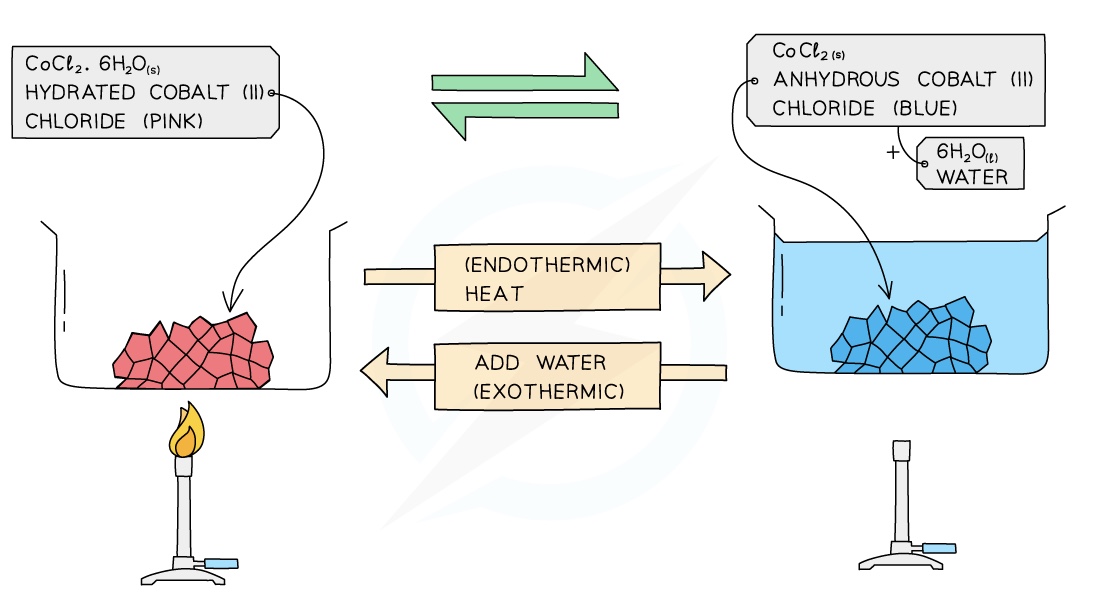
Hydration of Cobalt (II) chloride
When anhydrous blue cobalt(II) chloride crystals are added to water they turn pink and the reaction is reversible
When the cobalt(II) chloride crystals are heated in a test tube, the pink crystals turn back to the blue colour again as the water of crystallisation is lost
The form of cobalt(II) chloride in the crystals that are pink is known as hydrated cobalt(II) chloride because it contains water of crystallisation
When hydrated cobalt(II) chloride is heated, it loses its water of crystallisation and turns into anhydrous cobalt(II) chloride:
CoCl2.6H2O (s) ⇌ CoCl2 (s) + 6H2O (l)
Equilibrium and Le Chatelier
Equilibrium means there’s no overall change. The ammount of nitrogen hydrogen and ammonia remain steady But dynamic means there is continual Change.
In a Closed system, a reversible reaction reaches a state of dynamic equilibrium where the rate of forward reaction and backward reactions take place at the same time and rate (are equal) so there is no over all change.
The term Dynamic Equilibrium is usually shortened to equilibrium
Characteristics of reaction at equilibrium
It is dynamic e.g. the molecules on the left and right of the equation are changing into each other by chemical reactions constantly and at the same rate
The concentration of reactants and products remains constant (given there is no other change to the system such as temperature and pressure)
It only occurs in a closed system so that none of the participating chemical species are able to leave the reaction vessel

%%The reaction between H2 and N2 in the Haber process%%
Nitrogen and hydrogen react together to form ammonia
When only nitrogen and hydrogen are present at the beginning of the reaction, the rate of the forward reaction is at its highest, since the concentrations of hydrogen and nitrogen are at their highest
As the reaction proceeds, the concentrations of hydrogen and nitrogen gradually decrease, so the rate of the forward reaction will decrease
However, the concentration of ammonia is gradually increasing and so the rate of the backward reaction will increase (ammonia will decompose to reform hydrogen and nitrogen)
Since the two reactions are interlinked and none of the gas can escape, the rate of the forward reaction and the rate of the backward reaction will eventually become equal and equilibrium is reached:

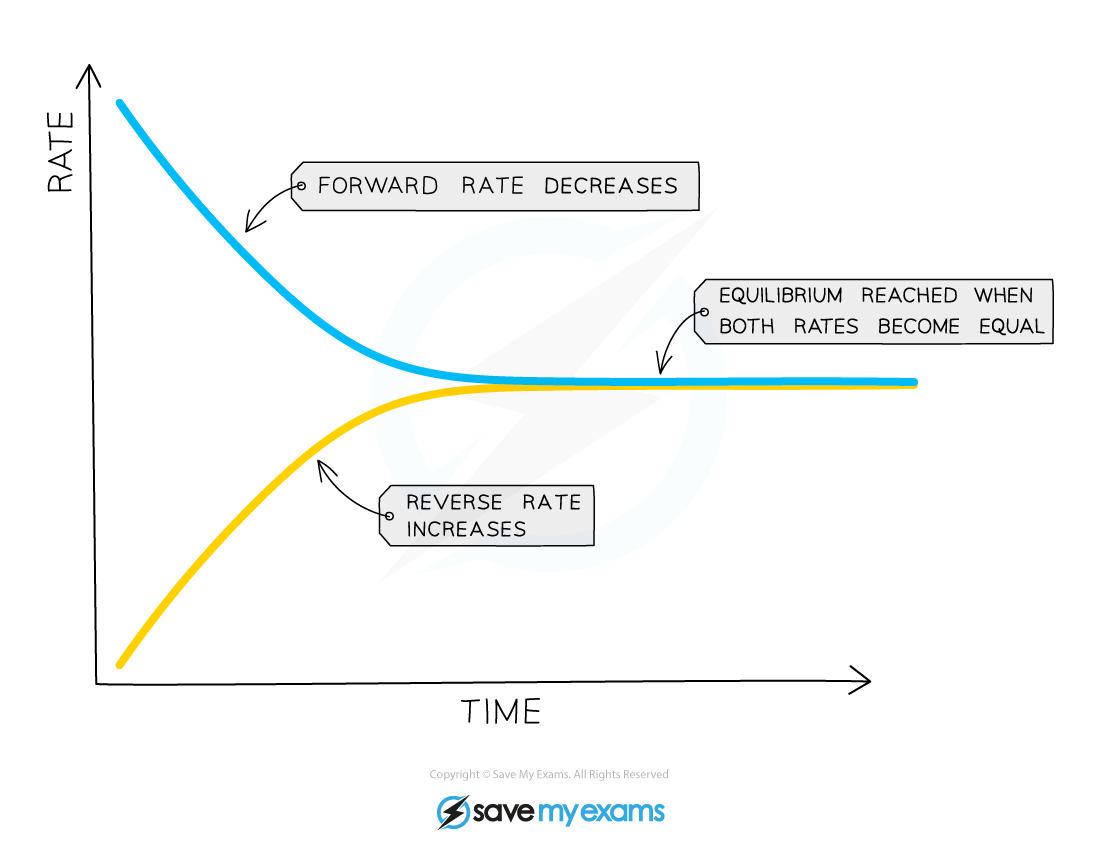
Position of Equilibrium
- Equilibrium position refers to the relationship between the concentration of reactants and products at the equilibrium state
- When the position of equilibrium shifts to the left, it means the concentration of reactant increases
- When the position of equilibrium shifts to right, this means the concentration of product increases
Effect of catalyst on equilibrium position
The presence of a catalyst does not affect the position of equilibrium but it does increase the rate at which equilibrium is reached
This is because the catalyst increases the rate of both the forward and backward reactions by the same amount (by providing an alternative pathway requiring lower activation energy)
As a result, the concentration of reactants and products is nevertheless the same at equilibrium as it would be without the catalyst
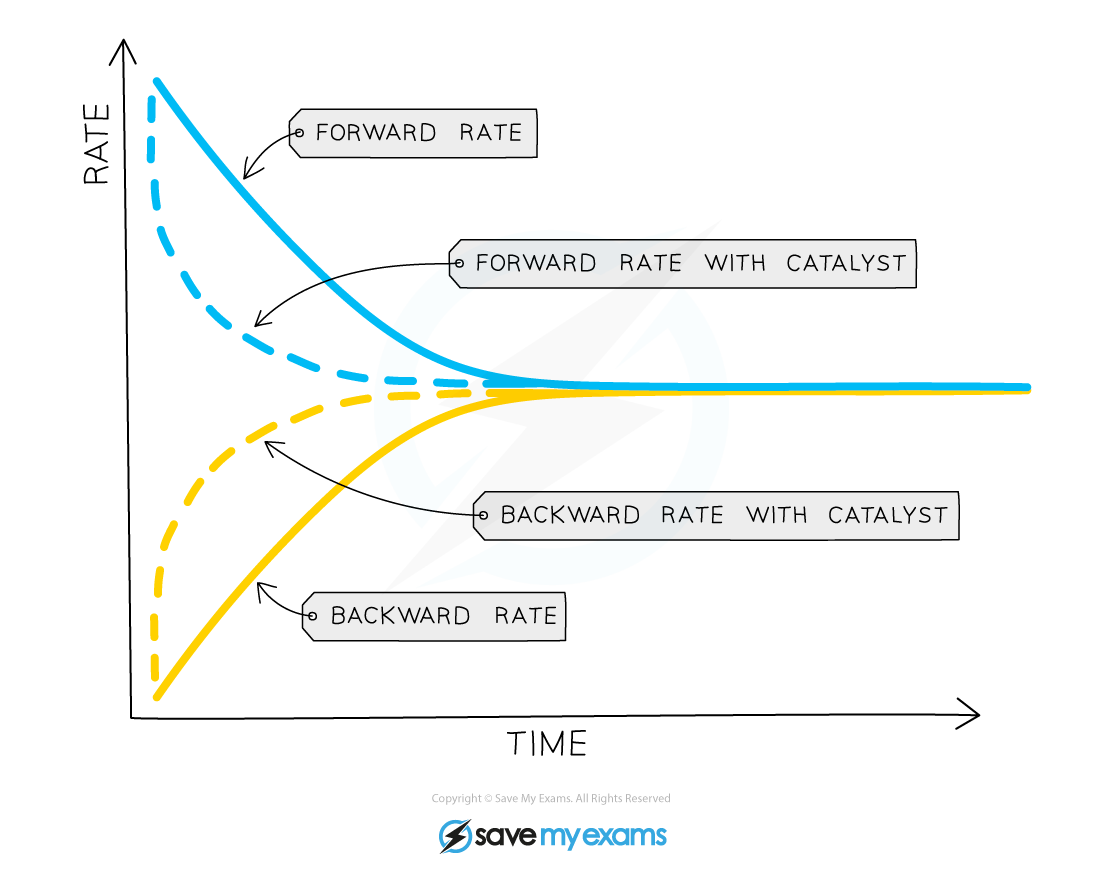
Le Chateliers Principle
Le Chatelier’s Principle states that when a change is made to the conditions of a system at equilibrium, the system automatically moves to oppose the change
The principle is used to predict changes to the position of equilibrium when there are changes in temperature, pressure or concentration
Effects of Pressure on Equilibrium
Example:
Nitrogen dioxide can form dinitrogen tetroxide, a colourless gas
2NO2 ⇌ N2O4 \n brown gas colourless gas
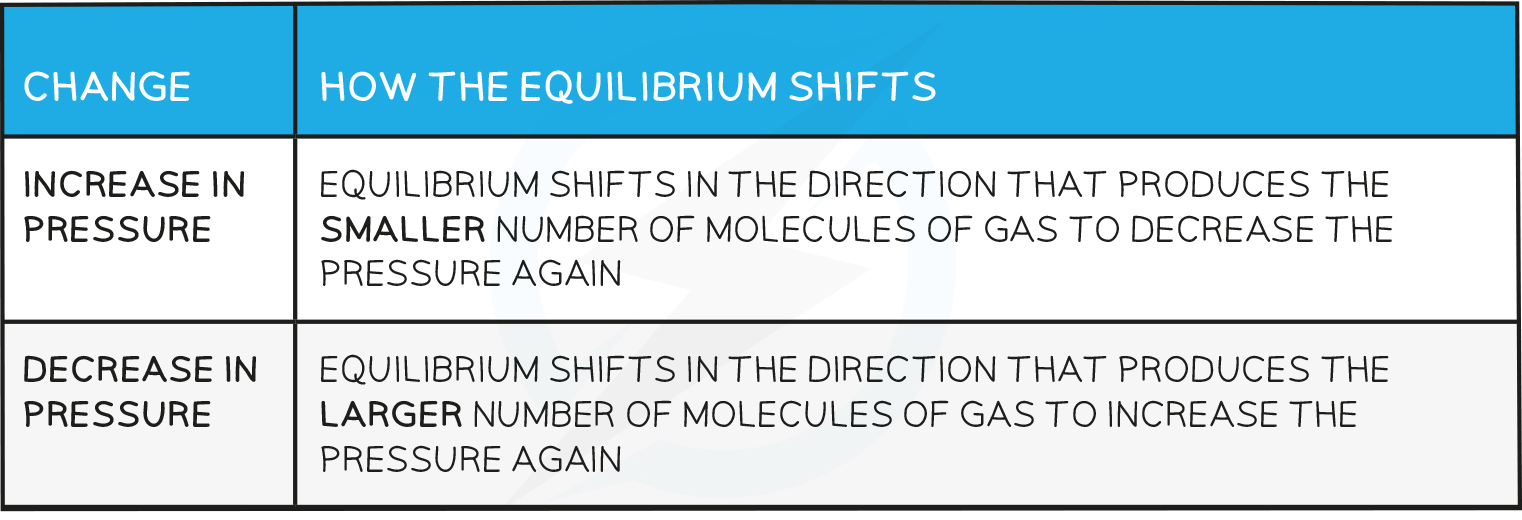
Predict the effect of an increase in pressure on the position of equilibrium:
Number of molecules of gas on the left = 2
- Number of molecules of gas on the right = 1
An increase in pressure will cause equilibrium to shift in the direction that produces the smaller number of molecules of gas
So equilibrium shifts to the right
The reaction mixture becomes paler as more colourless N2O4 is produced
Effect of Concentration on Equilibrium
Example: Iodine monochloride reacts reversibly with chlorine to form iodine trichloride
ICl + Cl2 ⇌ ICl3
dark brown yellow
Predict the effect of an increase in concentration on the position of equilibrium:
- An increase in the concentration of ICl or Cl2 causes the equilibrium to shift to the right so more of the yellow product is formed
- A decrease in the concentration of ICl or Cl2 causes the equilibrium to shift to the left so more of the darkbrown reactant is formed
