Chemistry Megaset
1/170
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
171 Terms
What's the difference between condensation, deposition, and sublimation?
Condensation = Gas into a liquid (think of water droplets on your mirror after you shower)
Sublimation = Solid into a gas (dry ice that produces Halloween Smoke)
Deposition = A gas directly into a solid (no liquid state in between)'; the opposite of sublimation

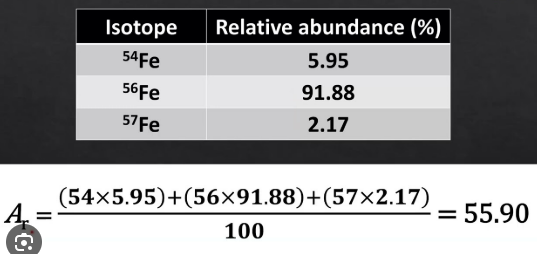
How do you calculate relative atomic mass?
turn the relative percentage abundance into decimals, then multiply the decimal by the isotopic mass:
Identify the metalloids:
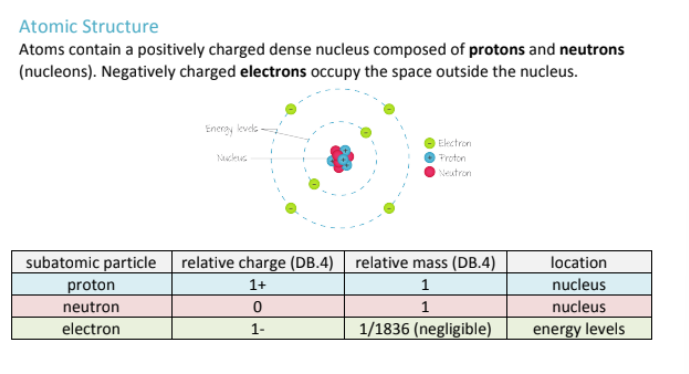
Where can you find protons on periodic table? what about electrons?
The atomic number on the periodic table represents the number of protons in an element's nucleus. The number of protons and electrons are equal unless it is an ion.
Each element has a unique atomic number, which determines its identity.
For example, hydrogen has an atomic number of 1, meaning it has 1 proton, while oxygen has an atomic number of 8, meaning it has 8 protons.
Since atoms are electrically neutral, the atomic number also tells you the number of electrons in a neutral atom.
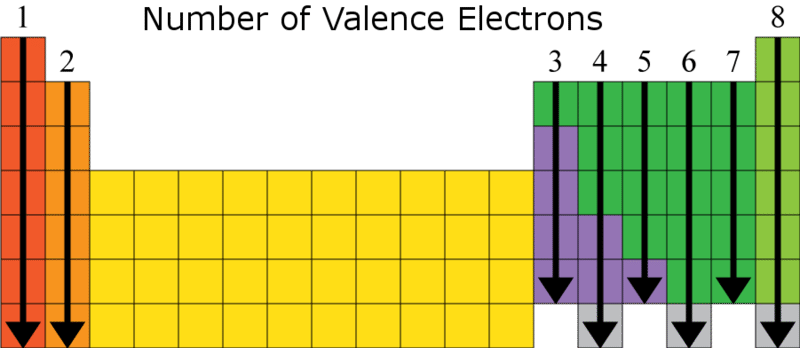
What do lewis structures show?
Lewis structures only show VALENCE electrons. The valence electrons are here:
Which statement best describes heat?
Heat is a quantity of energy transferred between particles
Distinguish between a mixture and a pure substance?
Mixtures = have two ore more compounds/elements mixed together, and are not chemically combined
Pure substances = are pure, made up of a single type of atom or a single type of compound; This can include elements (all made of the same atoms) OR compounds, atoms of different elements that have been combined in fixed ratios
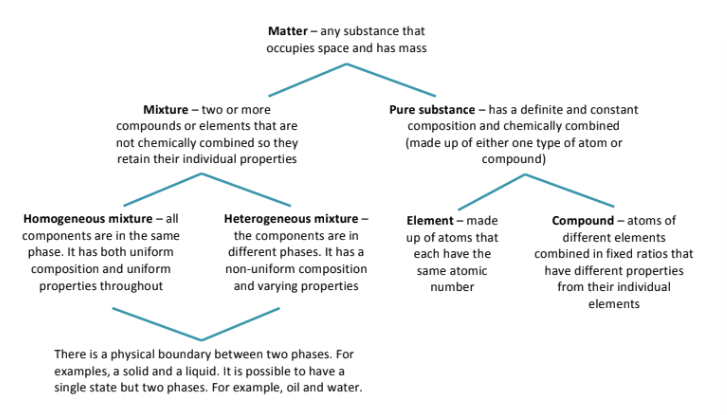
Describe the states of matter: solid liquid gas; Their volume, shape, forces, and particles
Solids =
have a fixed volume, a fixed shape, and cannot be compressed. Particles are held together tightly (are tightly packed together) & they vibrate in fixed positions
Liquids =
have a fixed volume, but take the shape of the container they are in, and cannot be compressed. The forces in a liquid are weaker than a solid, but stronger than a gas. Particles are constantly moving around.
Gas = has no fixed volume, no fixed shape, and CAN be compressed. There are no forces holding the particles of gases together, and the particles move around much faster than in liquids.
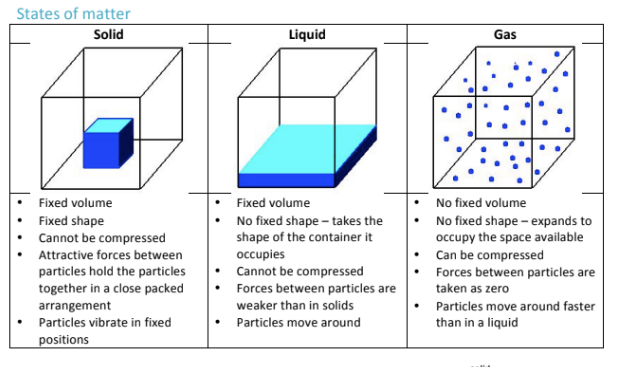
Which of the following is exothermic, and which is endothermic:
Melting, condensation, boiling, and freezing
Melting and boiling are both endothermic because they both require energy to be added in
Condensation and freezing are exothermic because they both release energy
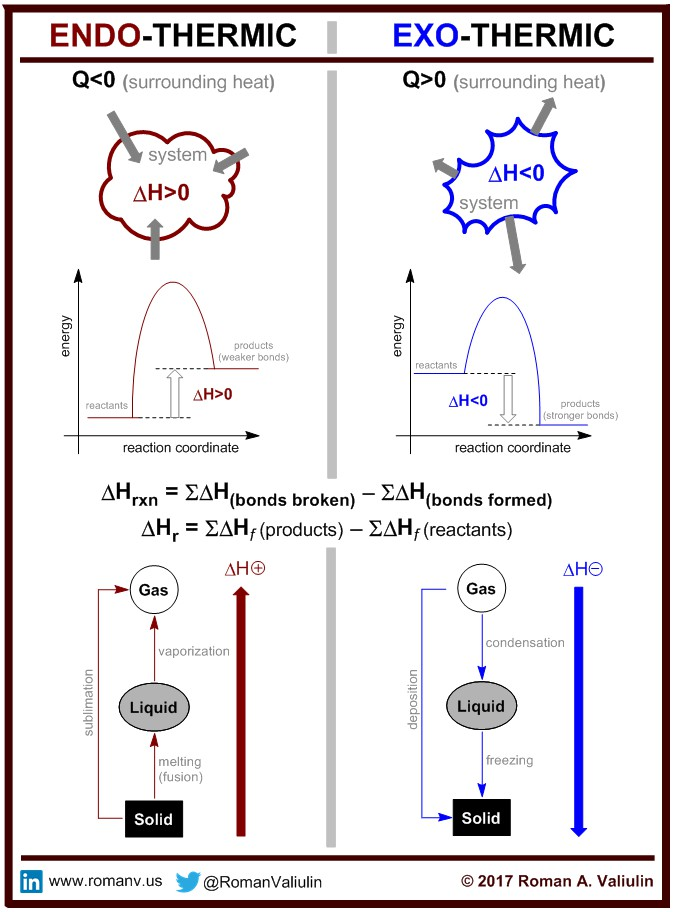
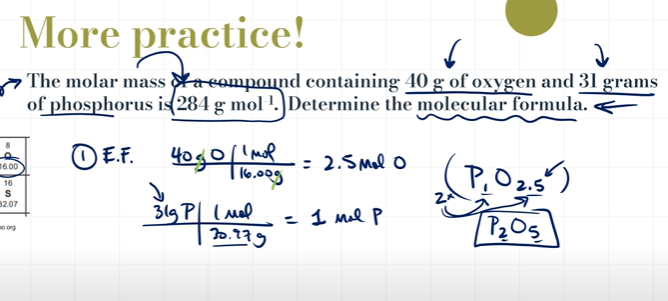
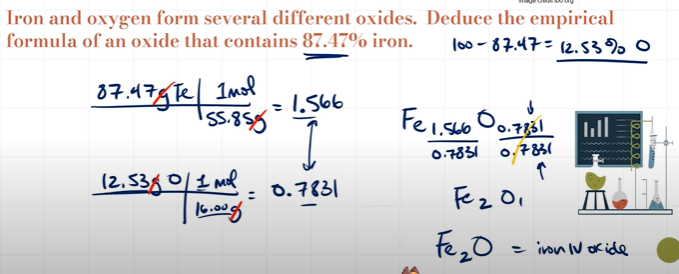
What is Empirical Formula and how do you calculate it?
Empirical Formula is the simplest whole number ratio of atoms

1. A compound is found to contain 63.52% iron and 36.48% sulfur by mass. Find its empirical formula.
For these questions, we assume 100 g of each

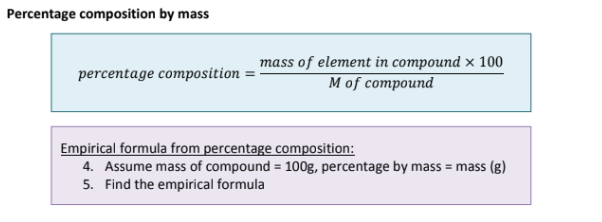
What is Percentage Composition and how do you calculate it?
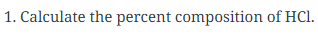
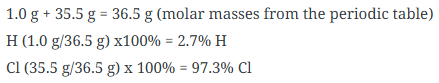
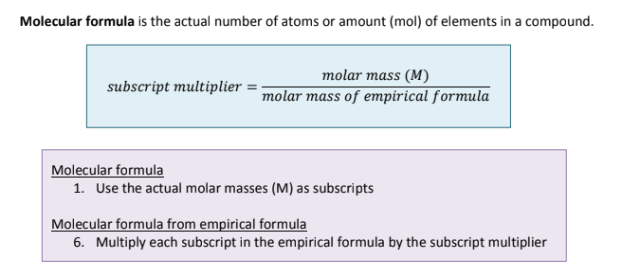
What about Molecular Formula?

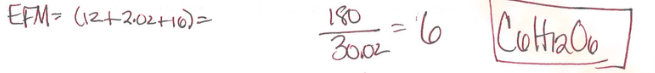



UNCOMPLETE: THE ANSWER IS ACTUALLY P4O10

Divide by the smaller # of moles
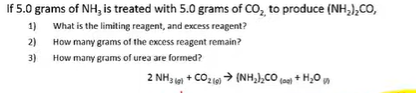
Answer to part one:
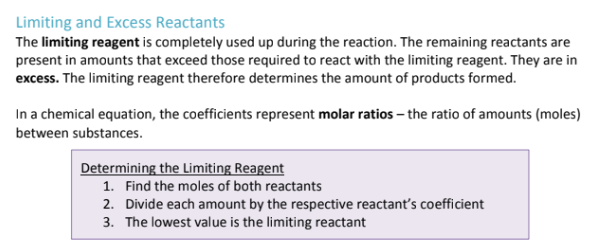
Question to ask yourself: If we were to use up all of the CO2, how much NH3 would we need?
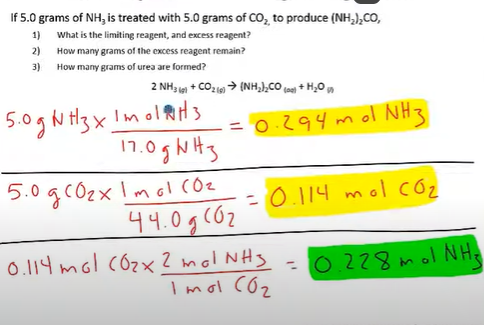

To figure out how much was produced, you have to use the limiting reactant to solve; because the limiting reactant is what allows us to only make a certain amount;

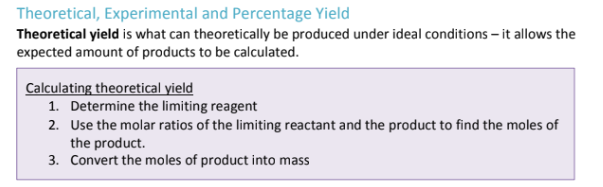
How do you calculate theoretical yield?
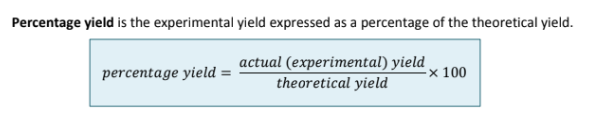
How do you calculate percentage yield?



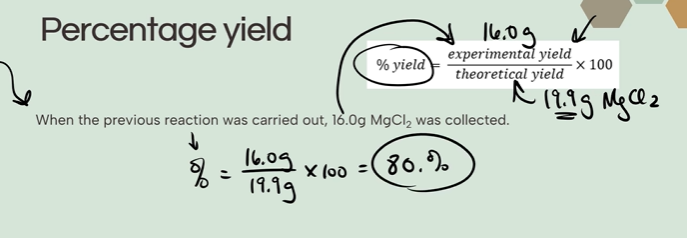
What does it mean when gases are at high pressure? What about at low temperatures?
High pressure = Think of a very small and tightly sealed container; particles are very close together
Low Temperatures = Gas particles move slower because they have less kinetic energy
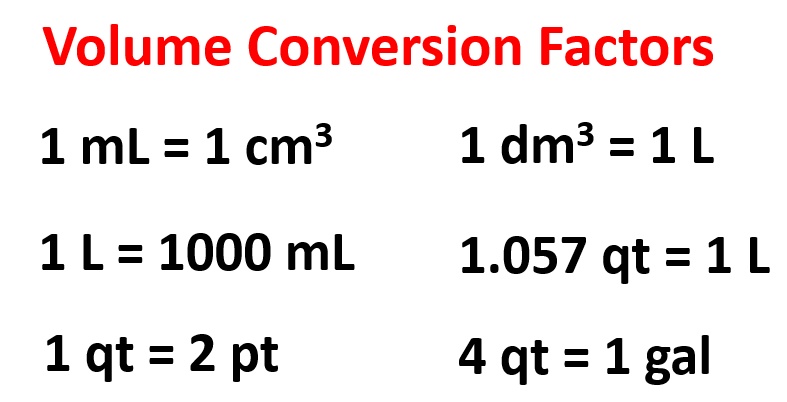
How do you convert from dm³ to cm³?
1 dm3 = 1000 cm3.
Mnemonic: “Deep Math³” (DM³) vs. “Cute Mice³” (CM³)
Imagine Deep Math³ (DM³) is a large brainy cube—it takes up a lot of space!
Now picture Cute Mice³ (CM³) running around in tiny cubes—you need 1000 of them to equal one big DM³ cube.
Charge of a proton, neutron and electron? Where are they found in an electron?
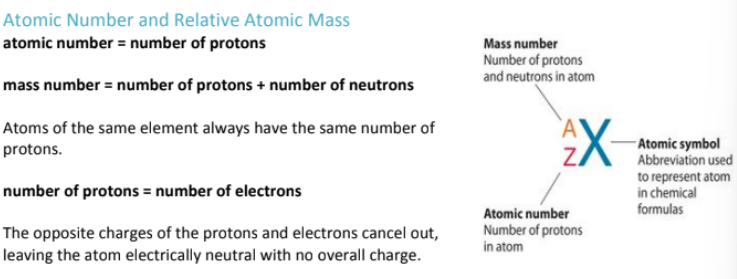
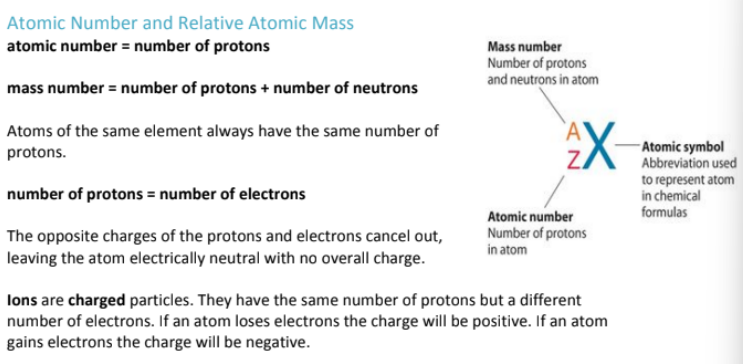
What are ions?
How many electrons are in each
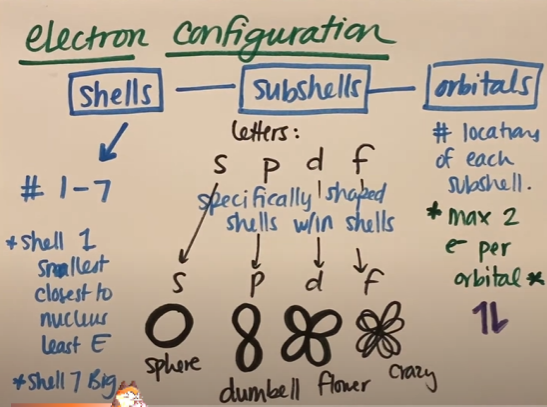
What’s the difference between shells, subshells, and orbitals?
Shells = The arrangement of electrons around a nucleus, numbered 1, 2, 3, 4 and so on; Each level contains different sublevels, increasing in complexity as you go higher.
Level 1 → Only s sublevel (2 electrons)
Level 2 → s and p sublevels (8 electrons)
Level 3 → s, p, and d sublevels (18 electrons)
Level 4 and beyond → s, p, d, and f sublevels (32 electrons at max)
Subshells (or Sublevels) = Groups of orbitals that make up shells; can either be s, p, d or f; The four main orbitals are:
s (1 orbital, holds 2 electrons) → Spherical shape
p (3 orbitals, holds 6 electrons) → Dumbbell shape
d (5 orbitals, holds 10 electrons) → More complex shapes
f (7 orbitals, holds 14 electrons) → Very complex shapes
Orbitals = clouds of electrons
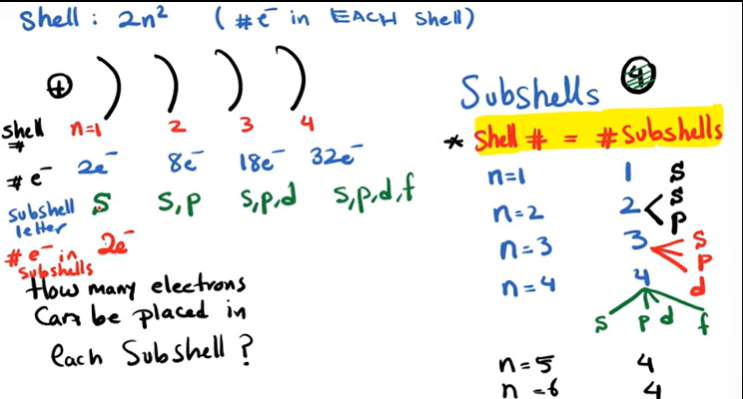
Define orbitals?
Orbitals are clouds of electrons; S orbitals are a simple sphere;
P orbitals can be on the x, y and z axis;
If asked to draw a P orbital, draw a dumbell going through the origin
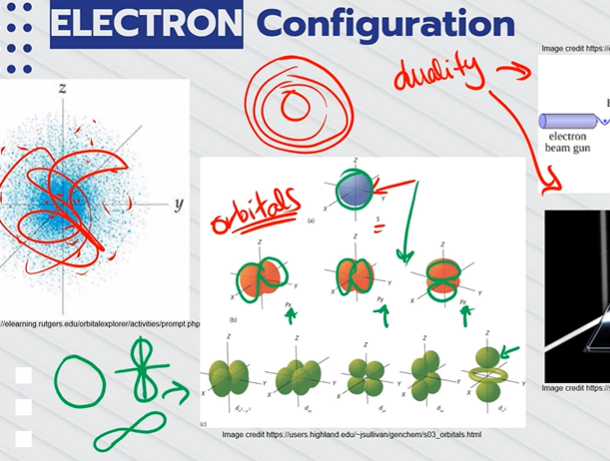
What are the two exceptions to electron configuration?
COPPER AND CHROMIUM;
The 4s sub level is filled before the 3d sub level, because they are more stable that way
What is the electromagnetic spectrum?
Electromagnetic Spectrum = EVERYTHING; Shows the full range of electromagnetic radiation; Visible light, x-rays, radio waves
Emission Spectrum = Shows how electrons are exicted from lower to higher energy levels
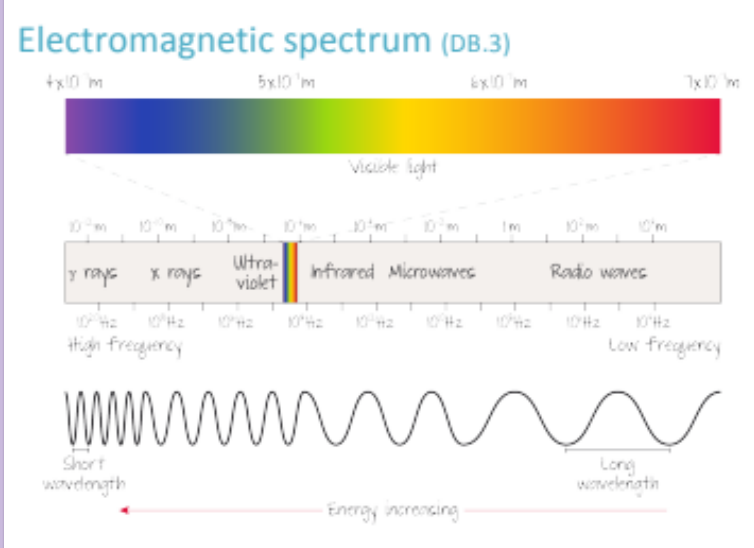
What is an emission spectrum? What are the two types of emission spectra?
An Emission Spectrum are produced when an electron is excited from a lower to a higher energy level; Whenever the electron falls down to a lower energy level, it emits a photon which is perceived as a different colored light;
THE TWO TYPES OF EMISSION SPECTRA:
CONTINUOUS SPECTRUM = Shows all wavelengths of visible light (a rainbow)
LINE SPECTRUM = Generally against a dark background, only shows distinct bright lines at specific wavelengths that correspond to the energy transitions of electrons in an atom (black with a few lines)
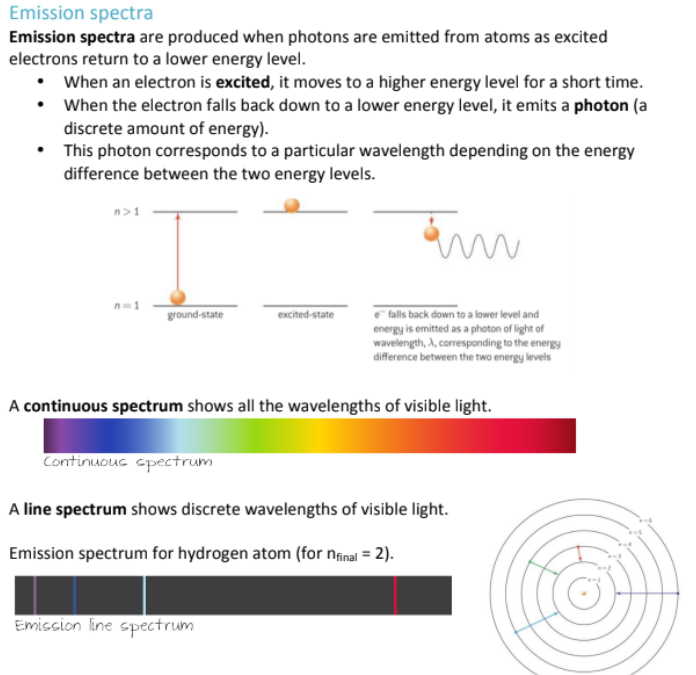
How is the size of a wavelength related to its frequency and energy?
Higher energy = higher frequency = shorter wavelength
Lower energy = lower frequency = longer wavelength
(to remember where these are located; just remember that humans can’t detect high frequency sounds, which are on the lower end of the electromagnetic spectrum)
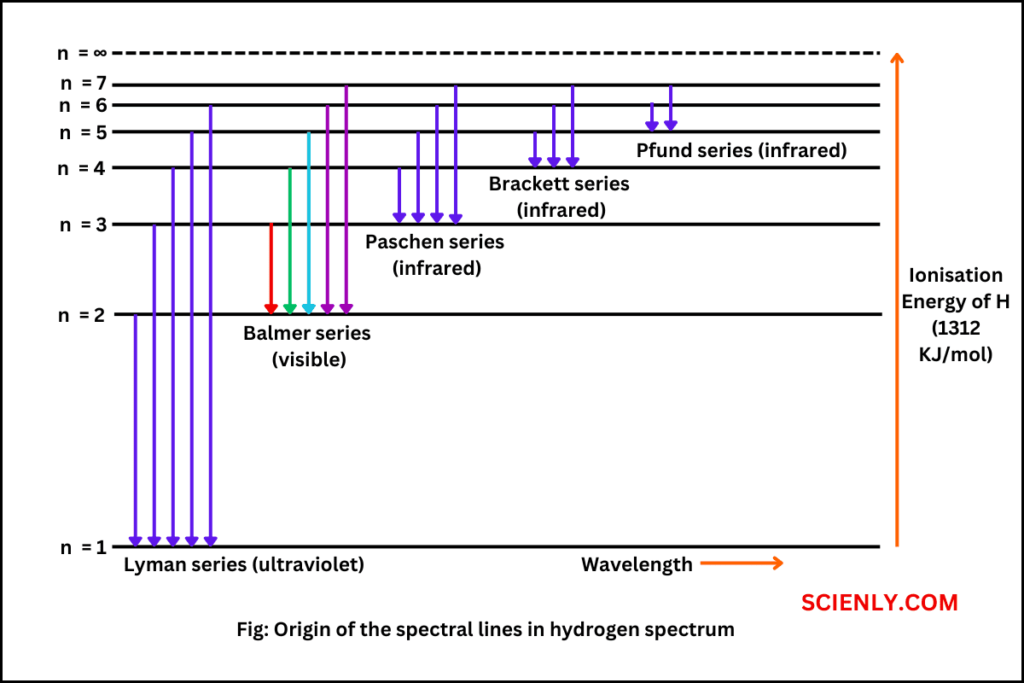
Draw the emission spectra for hydrogen? Where is visible light located?
LIONS = Lyman (from 6 to 1) (uv light that we cannot see, too high frequency)
BAKE = Balmer (Visible light) (from 6 to 2)
PERFECT = Paschen Series (from 6 to 3)
BROWNIES = Brackett Series (from 6 to 4)
(Corresponds to the same order on the electromagnetic spectrum)
What are the groups and periods?
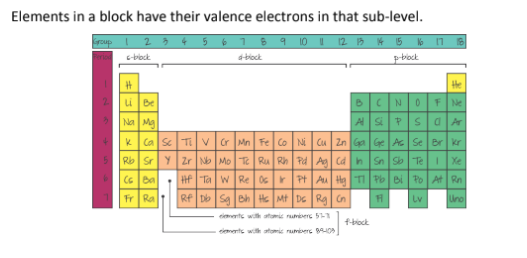
In which group/block are the Alkali Metals, Alkaline Earth Metals, Halogens, Noble Gases, Transition Metals, and Lanthanides and Actinides
Alkali Metals = Group 1
Alkaline Earth Metals = Group 2
Halogens = Group 7A
Noble Gases = Group 8A
Transition Metals = D block
Lanthanides and Actinides = F Block
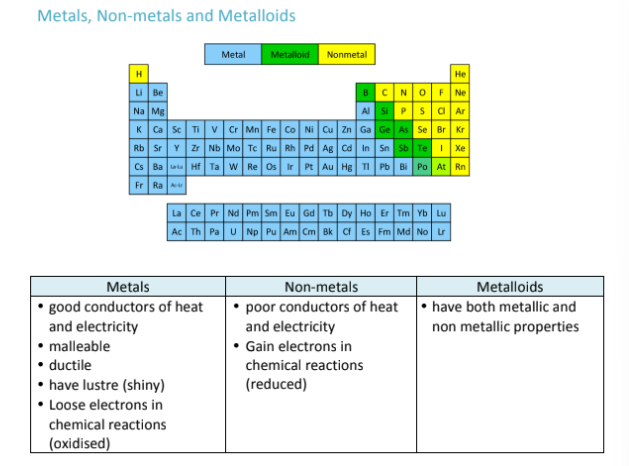
What are the properties of metals, metalloids, and non-metals?
What is the Trend for Atomic Radii on the Periodic Table?
It decreases across a period, but increases down a group.
As you go across a period, more protons are added, which increases the electrostatic attraction between the electrons and the protons in the nucleus; causing the electrons to be more tightly held;
As you go down a group, the number of shells increases, and this creates electron shielding, because electrons are further away from the nucleus, decreasing their electrostatic attraction; therefore atoms are larger and electrons are held less closely;

What is the trend for Ionic Radii across a period and down a group?
Across a period, the ionic radius decreases for Metals which form Cations (lose electrons), because they are LOSING ELECTRONS, becoming more positive, meaning that there is less electron shielding, and the electrons are more strongly attracted to the protons in the nucleus of atom, pulling it closer together.
Across a period, the ionic radius INCREASES for Non-metals that form Anions (gaining electrons), because the gain of electrons means that there is more electron shielding, and a lower electrostatic attraction between the electrons and the nucleus, meaning electrons are held less closely together, making it larger.
Down a group, the ionic radius increases for both Cations and Anions because more electron shells are added, increasing the distance of the electrons from the nucleus, leading to a lower electrostatic attraction because of electron shielding and a larger ionic size.

What is First Ionization Energy? What is the trend for Ionization Energy across a period and down a group?
First ionization energy is the minimum amount of energy required to move one electron from an atom
Across a Period: Ionization energy Increases, because the number of protons increases, and the electrostatic attraction between the nucleus and the electrons are greater; meaning electrons are held much tightly/closer together, requiring more energy to remove an electron.
Decreases down a Group, because you have more shells, meaning more electrons are further away from the nucleus, a lower electrostatic charge, and a greater atom that makes it easier to remove an electron

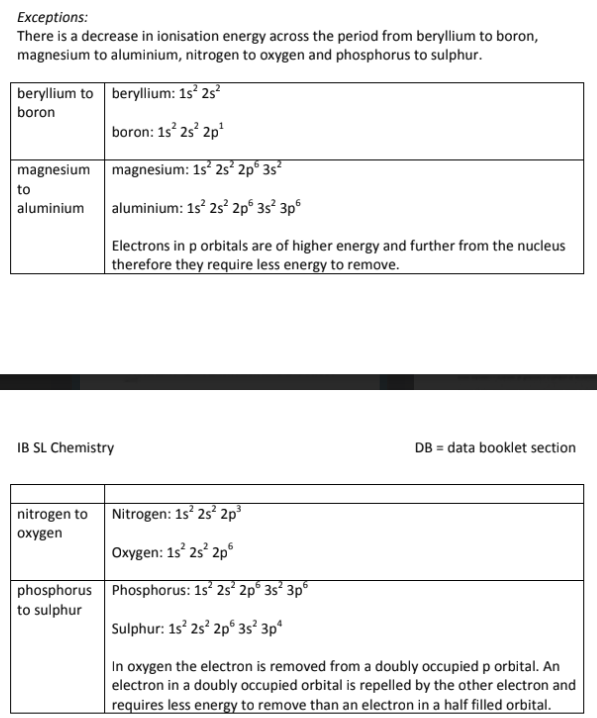
What is the Exception for Ionization Energy?
There is an exception across the period from Beryllium to boron (or from Group 2A to Group 3A)
The Group 2 elements have an outer electron in the s-orbital, which is closer to the nucleus and more tightly held;
While Boron and Aluminum have their outer electrons in a p-orbital which causes the electrons to be slightly further away from the nucleus, resulting in more shielding, and therefore a lower ionization energy;
Think of s vs. p orbitals: p is further out, so it's easier to take an electron away!
What is the trend for Electron Affinity across a period and down a group?
Electron Affinity is the attraction between an incoming electron and the nucleus, the stronger the attraction, the more energy is released when an electron is added
Electron Affinity Increases across a period (with the halogens actually have the highest electron affinity or attraction to electrons, because its so small, so its nucleus has a strong attraction to incoming electrons)
It decreases down a group as elements get larger and the electrostatic charge is lesser

Do Metals or Non-metals have a greater ionization energy? Why?
Non metals have a greater Ionization energy than metals because they have more protons in their nucleus, meaning these protons and the electrons in its shells are more strongly attracted to each other; a higher electrostatic attraction, and are therefore held more closely together, resulting in a greater ionization energy.

What are metal and non-metal oxides?
Metal oxides are a combination of a metal with oxygen, non-metal oxides are a combination of a non-metal with oxygen

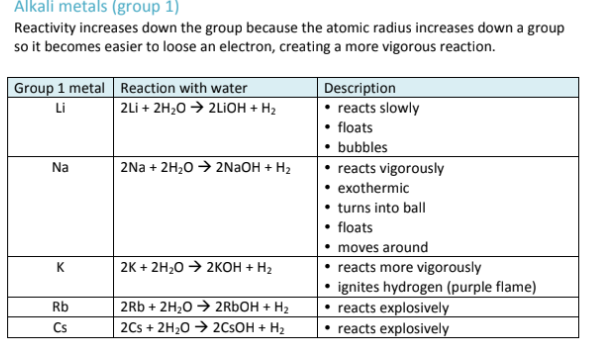
What is the reactivity of the Alkali Metals like?
The Alkali Metals are in Group 1, and their reactivity increases going down the group, because they have a greater atomic radius, making it easy to lose an electron and take part in chemical reactions.
remember: This trend only applies because metals DONATE electrons
What is the reactivity of the Halogens like?
The Halogens become less reactive down a group; Because their atomic radius increases, meaning there is a weaker electrostatic attraction between the protons in the nucleus and the electrons; But as a result of this lower electrostatic attraction, electrons are harder to GAIN
remember: This trend only applies because non-metals GAIN electrons (they don’t donate electrons)

What is the melting and boiling point like for Halogens?
Melting & Boiling points increase for halogens going down the group because as the size of the atom increases, london dispersion forces (weakest Van der waal forces) become stronger and require more heat to break.
How do you convert from m to nm?

How are Cations & Anions Formed?
Cations = Are formed by metals losing valence electrons (oxidation)
Anions = Are formed by metals gaining valence electrons (reduction)
How do you find the # of protons on periodic table:
How do you write the formula for an ionic compound; Like Aluminum Trioxide

Hydroxide
OH-
Nitrate
NO3-
Hydrogen carbonate
HCO3-
Carbonate
CO32-
Sulfate
SO42-
Phosphate
PO43-
Ammonium
NH4+
How can you recognize an Ionic bond between a metal and non-metal based on its electronegativity? Are electrons shared?
They have an electronegativity difference of 1.8 or greater
Electrons are not shared; they are donated by the metal to the non-metal (non metal is reduced, metal is oxidized)

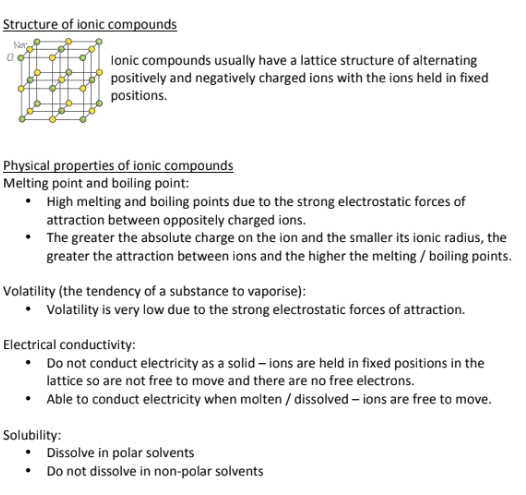
Describe the structure and physical properties of Ionic Compounds
Ionic Compounds Form Crystal lattice structures of alternating positive and negatively charged ions. The ions are held in fixed positions;
Ionic compounds (think of salt) are:
Brittle
Have a very high melting and boiling point because of their strong electrostatic forces of attraction
The smaller the ion, the greater its electrostatic forces and the higher its melting and boiling point
Has LOW Volatility, and is highly unlikely to vaporize into a gas because of its strong electrostatic forces
Doesn’t conduct electricity unless its dissolved in water (salt conducts electricity well when placed in water); Doesn’t conduct electricity well in its lattice because there is no room for the electrons to move around and conduct electricity
Dissolves in water, making it polar (because water is polar) (like dissolves like)
How are covalent bonds formed?
What do shorter bonds in covalent compounds mean?
Covalent bonds are formed between two non metals that have a shared pair of electrons and the attraction between their positively charged nucleus.
The shorter the covalent bond, the greater the strength
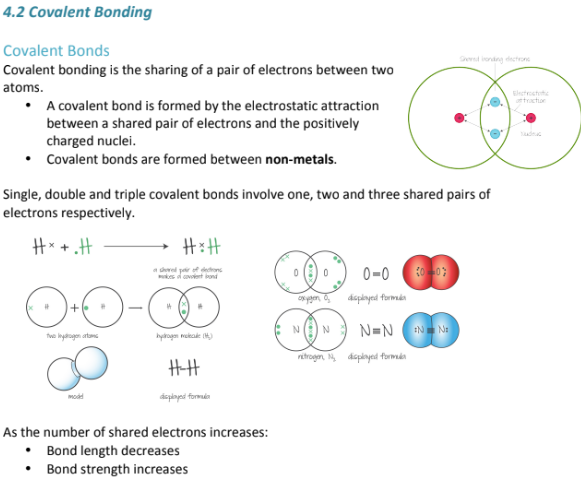
What is bond polarity all about? What does it mean to have polar and non-polar covalent bonds?
The difference in electronegativity; Polar Covalent bonds have one atom that is more electronegative than the other, resulting in a slightly negative charge on one atom and a slightly more positive charge on the other. Also in polar covalent molecules, the electrons are usually shared unequally, and you tend to see a lot less symmetry.
What are the properties of covalent compounds like?
They are the exact opposite of the properties of ionic compounds;
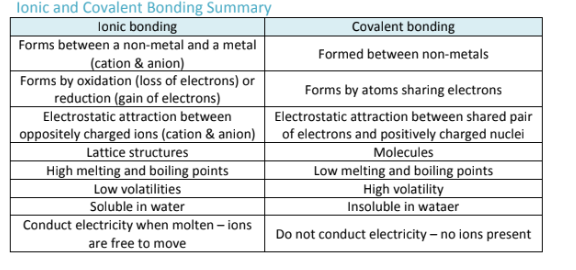
How are lewis structures different from bohr structures?
They only show the valence electrons in a covalently bonded molecule
Which elements violate the octet rule?
Hydrogen, Boron, Beryllium, Aluminum
HBBA

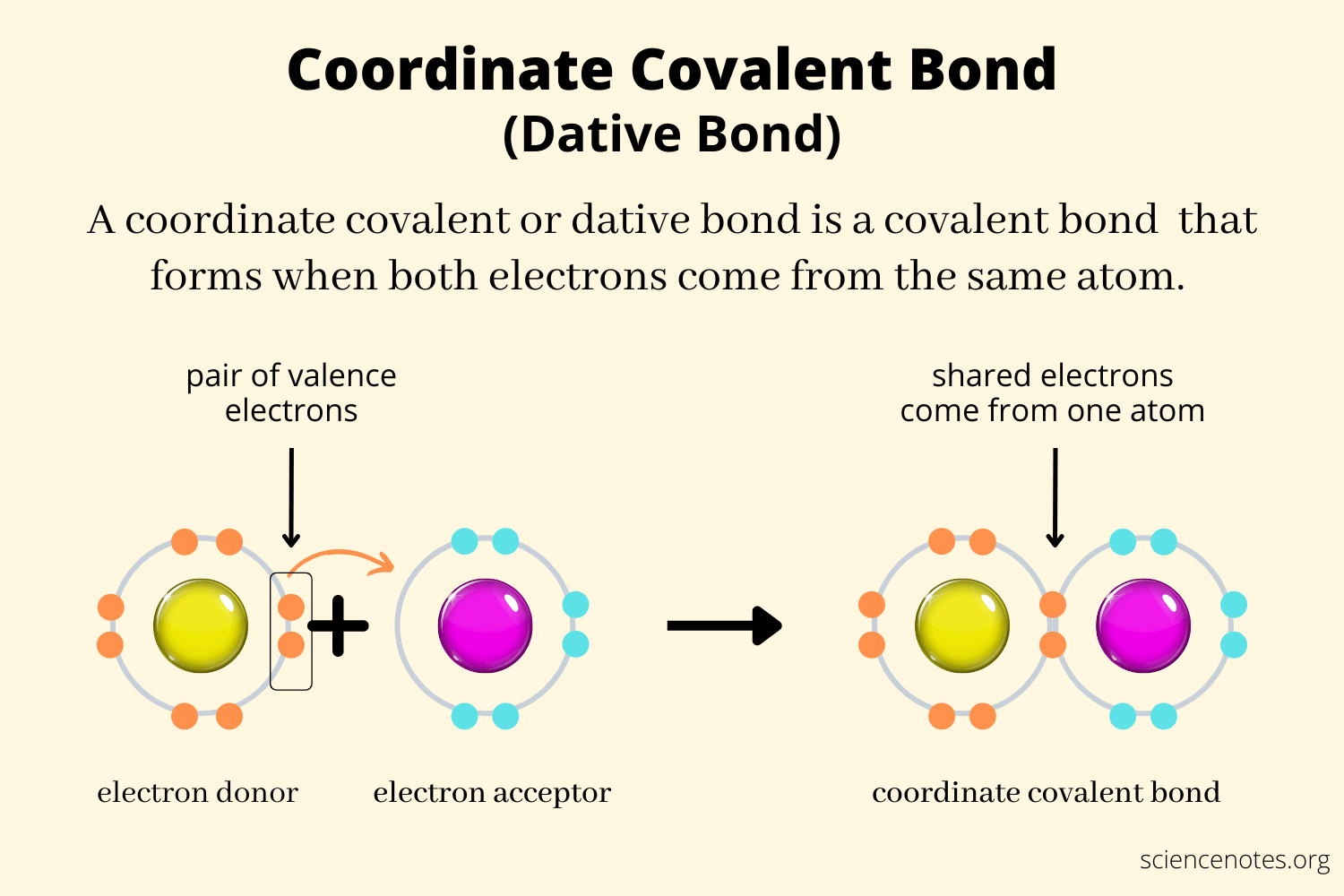
What happens in Coordinate Covalent bonding?
A covalent bond where the shared pair of electrons comes form a single atom;
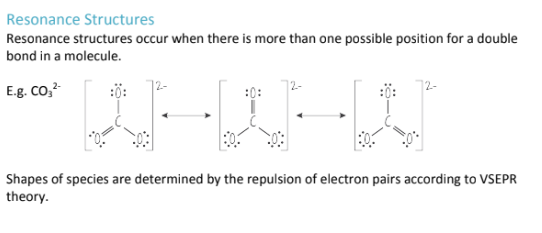
What are resonance structures?
Resonance structures are drawn using LEWIS diagrams; And they show the different possible positions for a double bond in a molecule
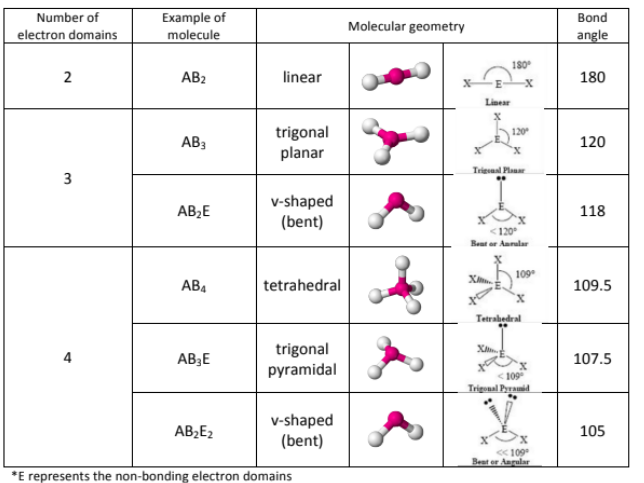
In VSEPR, what’s the difference between electron domain geometry and molecular geometry? What effect do electrons have on VSEPR structure?
Electron Domain Geometry = Takes into consideration the Lone pairs of electrons
Molecular Geometry = Does not take into consideration the lone pairs;
More electrons pushes the electrons closer together, reducing their bond angle;
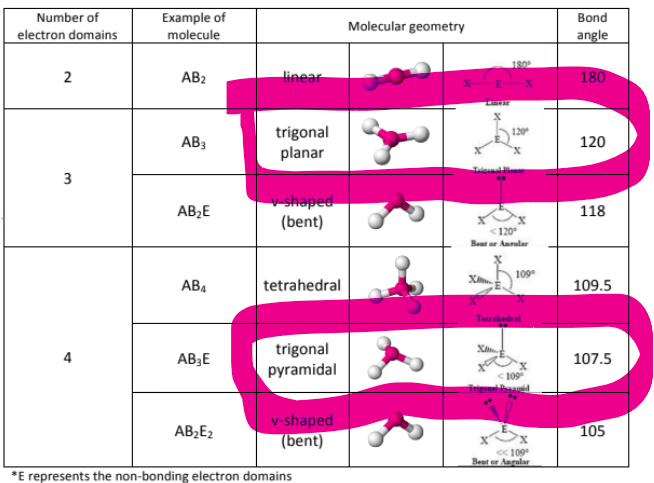
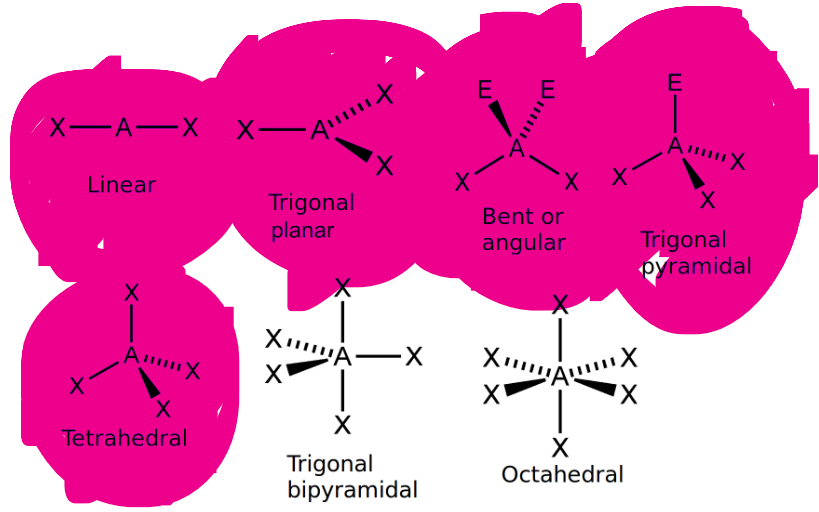
How is Trigonal Planar Different from Trigonal Pyramidal?
Trigonal planar is just when you have three things bonded to a central atom, AND NO LONE PAIRS, it has an angle of 120
Trigonal Pyramidal is when you have three things bonded to a central atom, AND AN EXTRA LONE PAIR that causes the electrons to be pushed closer together, reducing the bond angle to 107.5
What is the difference between the two forms of bent, and linear?
Linear:
has only two things attached to a central atom in a straight line WITH NO LONE PAIRS, meaning it just has an angle of 180 degrees
Bent shape #1:
has two things attached to the central atom, and ONE LONE PAIR that causes the electrons to be pushed slightly closer to each other, reducing the angle from 180 to 118
Bent shape #2:
has two things attached to the central atom, and TWO LONE PAIRS that causes the electrons to be pushed slightly closer to each other, reducing the angle from 180 to 105
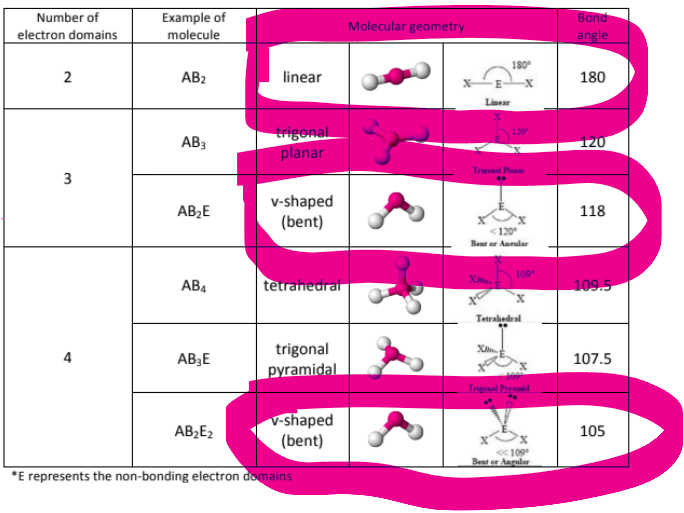
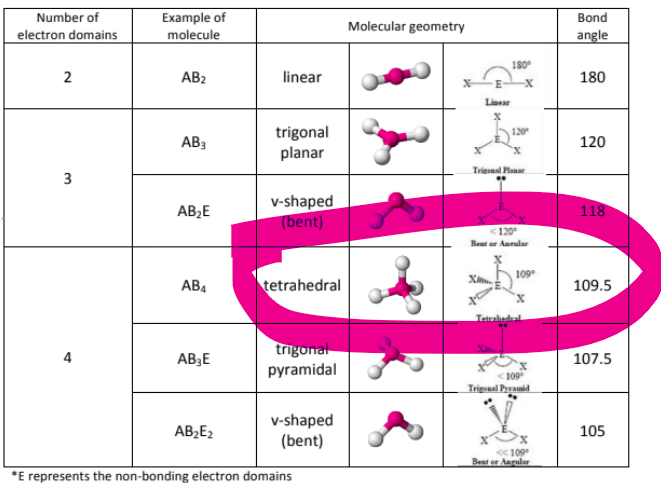
What about tetrahedral? How many different forms of tetrahedral are there?
There’s only one form of tetrahedral, and it has a bond angle of 120 degrees
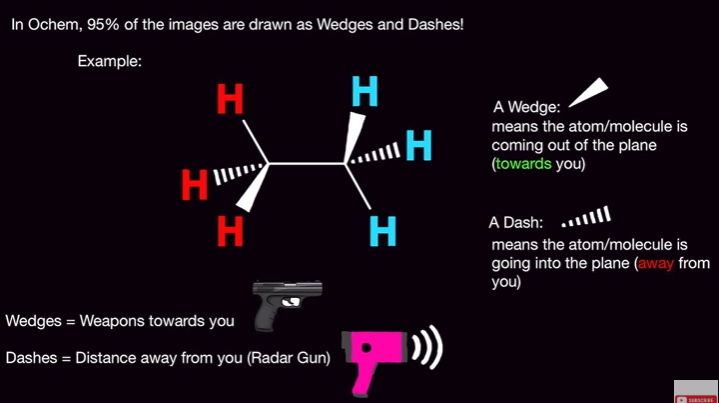
What do the wedges and dashes mean in wedge-dash notation?
Wedges = are coming out towards you (think of a solid weapon)
Dashes = are away from you (radar gun)
Can you draw the VSEPR structures with the wedge and dash notation?
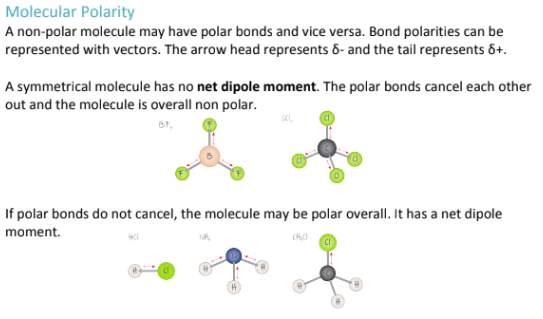
What does dipole dipole mean?
No net dipole means you have polar bonds that cancel each other because the entire molecule is symmetrical, making the overall molecule non-polar;
Dipole-Dipole means you don’t have polar bonds that cancel each other out, because the molecule is not symmetrical (in terms of shape and electronegativity), and therefore, the molecule is polar overall.
What are Network Covalent Structures?
A structure where atoms are bonded together through strong covalent bonds in a repeating network.
THEY ARE NOT SIMPLE MOLECULAR COMPOUNDS, because you have one large interconnected lattice of atoms covalently bonded together;
Repeating Lattices naturally form in Ionic compounds between oppositely charged ions, but the same thing cannot be said about covalent structures; Only some covalent structures exhibit lattice structures.
Which covalent compounds form giant covalent networks? Describe their structure, geometry, and properties
Diamond
Forms a giant covalent network where each carbon is covalently bonded to 4 other carbons
Tetrahedral Geometry, with an angle of 109.5 degrees
Strong Covalent Bonds that give it a very high melting and boiling point, is extremely hard, is a poor conductor of electricity because it has no delocalized electrons, and it is colorless/transparent
Graphite
Forms a giant covalent network where each carbon is bound to 3 other carbons
Trigonal Planar Geometry with an angle of 120 degrees
Its layers of hexagonal rings are connected by weak intermolecular forces (London Dispersion Forces) between each other
Has very strong covalent bonds, that give it a high melting and boiling point. Unlike diamond, it has weak IMFs inbetween its layers, resulting in a soft texture because layers can slide over each other. It is dark grey and shiny, and is a good conductor of electricity because it has delocalized electrons in its layers.
GrapheneA single layer of graphite (hexagonal), that is one atom thick, and contains one carbon bound to 3 carbons
Because its only a single layer, and not multiple layers, it does not have any intermolecular forces
Trigonal Planar Geometry with a 120 degree bond angle
Only contains strong covalent bonds, that give it a high melting and boiling point. It has delocalized electrons that allows it to conduct heat and electricity well
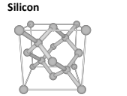
Silicone Dioxide (aka Quartz)
Forms a giant covalent network where each silicon atom is bound to 4 oxygen atoms.
Linear Geometry, with a 180 degree angle
It has strong covalent bonds, which give it a high melting and boiling point. it is also transparent and gray, and is not a good conductor of electricity because it has no delocalized electrons.

What are allotropes?
Different forms of the same element, in the same state (solid)
Do intermolecular forces only exist in ionic compounds?
No, intermolecular forces ONLY exist in covalent compounds, not ionic ones!
Why?
Intermolecular forces are forces of attraction between molecules.
Since ionic compounds do not form molecules, they do not have intermolecular forces.
Instead, ionic compounds have electrostatic forces (ionic bonds) between oppositely charged ions in their lattice structure.
2. Covalent compounds (molecular substances) do have intermolecular forces, which determine their physical properties like boiling and melting points.
What’s the difference between a compound and a molecule?
Compounds = Are made up of one or more types of atoms; Can be found in both Covalent and Ionic bonding, because covalent bonds can also form compounds with two different non-metals
Molecules = Are only made up of a single type of atom (all from the same element); This can only exist in covalent compounds, where you can have the same element bonded to the same element through covalent bonds.
This doesn’t exist in ionic compounds, because they require two different atoms from two different elements that have an electrostatic attraction to each other
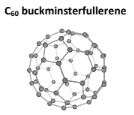
What are some other allotropes of carbon that DO NOT exhibit covalent networks? What about silicone?
C60, Buckmisterfullerene:
Is just a simple molecular solid that DOES NOT exhibit a giant covalent network
It contains 20 hexagons and 10 pentagons arranged in a ball, where each carbon atom is bound to three other carbons
It has Trigonal Planar Geometry, with a bond angle of 120
It has weak London Dispersion Forces between its molecules, but it also has strong covalent bonds holding the hexagons/pentagons together
It can conduct electricity because it does contain delocalized electrons, but not as well as graphite
2. SiliconeEach silicone atom is bound to 4 other silicone atoms
Tetrahedral geometry with an angle of 109.5 degrees
It has strong covalent bonds, giving it a high melting and boiling point; But it does not conduct electricity because it has no delocalized electrons
Where can you find London Dispersion Forces? Why are they formed
London dispersion forces are THE WEAKEST form of IMFs found between all atoms and molecules (made up of a single type of atom).
They are formed because of temporary shifts in the concentration of electrons on one side of an atom or molecule, which gives it a slight negative charge and the other side a slightly more positive charge; (a temporary dipole)
A molecule with a temporary dipole can induce a dipole in a neighboring molecule
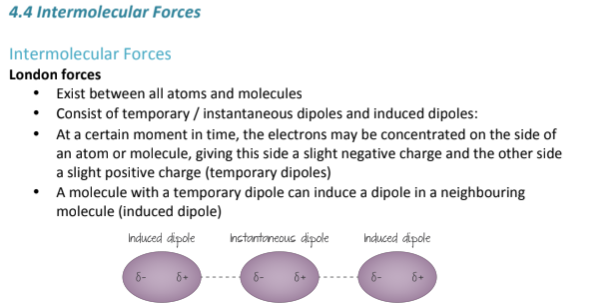
What increases the strength of London Dispersion Forces?
Larger Surface Area & Molar Mass
What are dipole-dipole forces? Where do they exist?
Dipole-Dipole forces exist in Polar molecules. It is caused by a permanent difference in electronegativity between two atoms. They are stronger than London Dispersion Forces, but weaker than Hydrogen Bonding.
(An uneven distribution of charge/electronegativity in polar molecules)
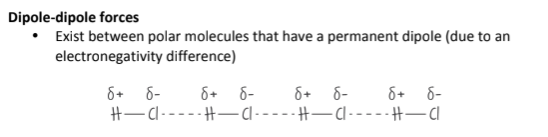
What are “Van Der Waal Forces”
A term that encompasses both London Dispersion Forces & Dipole-Dipole Forces & Induced Dipoles

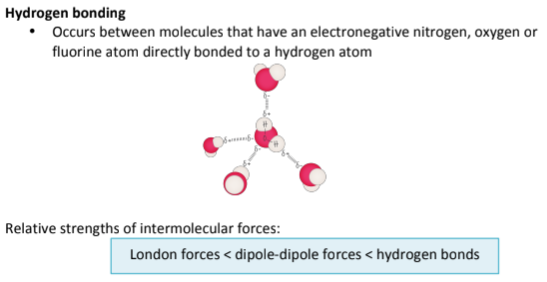
What is hydrogen bonding?
Bonds between molecules (one type of atom) that have an Electronegative Nitrogen, Oxygen, or Fluorine atom bound to a Hydrogen atom. It is the Strongest Intermolecular Force.
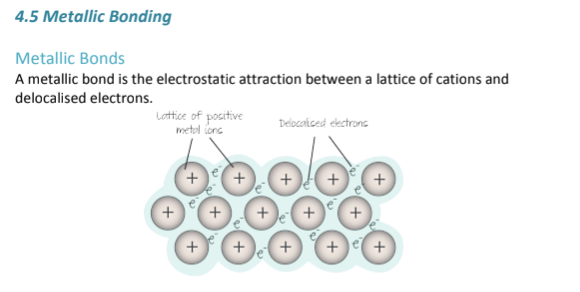
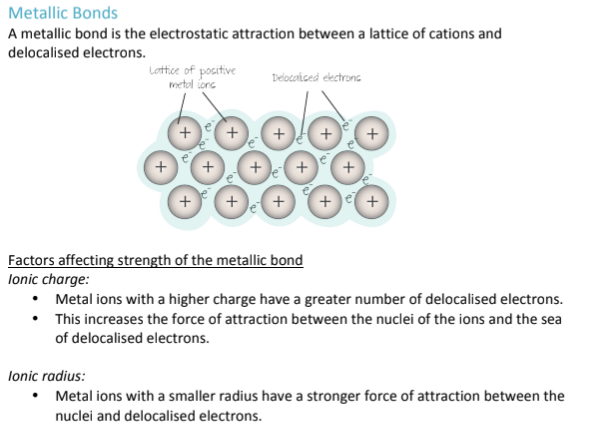
What is metallic bonding? Where does it occur?
Metallic bonding is a type of bond that only occurs in Ionic COMPOUNDS (more than one type of atom); It is the electrostatic attraction between a sea of electrons and Metal Cations (metals that have lost electrons, been oxidized)
What factors influence the strength of a metallic bond?
Ionic Charge
The higher the charge of the metal ion, the more electrons it originally had to use (ex. Na+ vs Mg2+); Mg2+ definitely lost more electrons, which means more delocalized electrons are floating around in the sea of electrons surrounding the metal cations; therefore, there is a greater electrostatic attraction between the nucleus of the ions and the sea of electronsIonic Radius
A smaller ionic radius means that electrons are held more closely to each other, increasing the electrostatic charge and strength of a metallic bond
What are some of the properties of metals?
Metals are:
Good Conductors of Heat & Electricity because of delocalized electrons
Are highly malleable and ductile (can be bent into different shapes) because they have layers of ions that can slide over one another and contain delocalized electrons
Metals with stronger metallic bonds have higher melting and boiling points
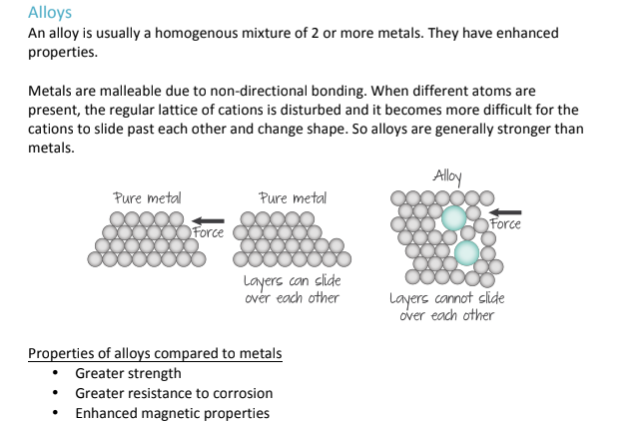
What are Alloys? What are some of the properties of alloys?
A homogenous mixture of 2 or more metals put together, that give them enhanced properties; Because they are a mixture, the structure becomes irregular, and this disrupts the movement of the metal cations, making it harder for layers to slide past each other, and making them less malleable/ductile; However, as a consequence of them being less likely to change shape, they are also much stronger than metals, more resistant to corrosion, and have better magnetic properties.
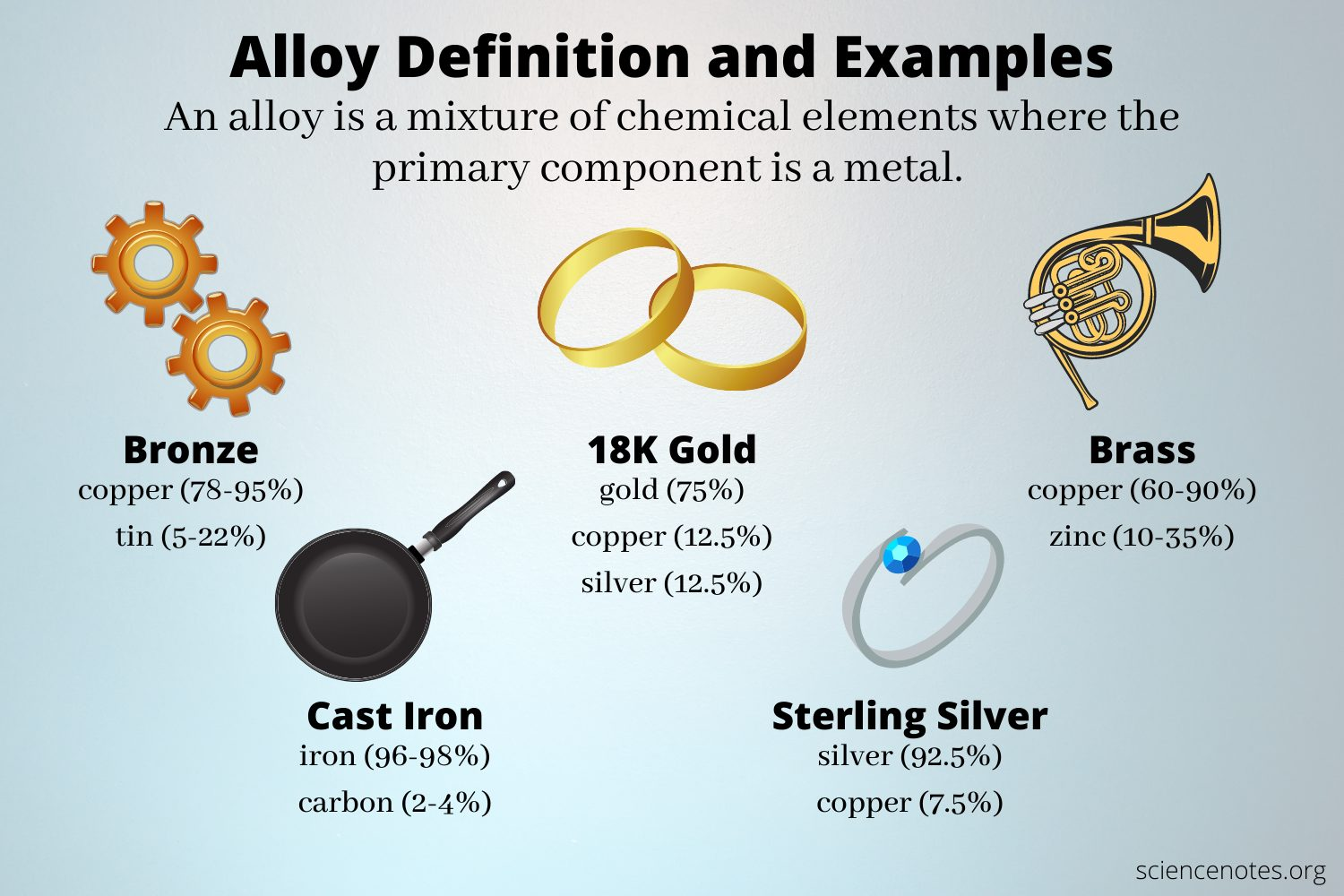
Can you give an example of different kinds of alloys?
Steel = Iron + Carbon
Stainless Steel = Iron, Chromium + Nickel
Brass = Copper & Zinc
Bronze = Copper & Tin
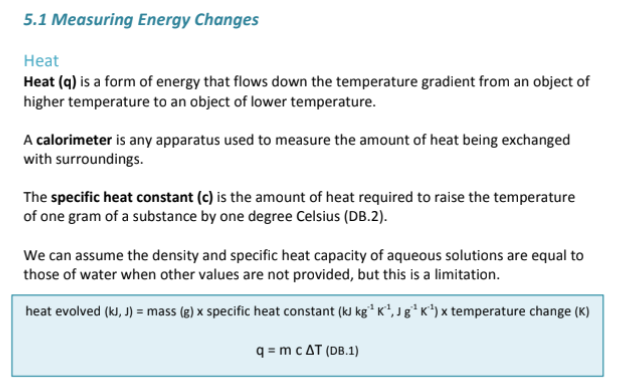
Define what a calorimeter is?
A device used to measure the amount of heat that is exchanged with the surroundings
Define specific heat capacity
The amount of heat required to raise the temperature of one gram of a substance by one degree celsius
Define temperature? Define Heat?
How do you convert to kelvin from celsius?
Temperature = Measures the average kinetic energy of particles
Heat = A form of energy that is transferred between particles
add 273 to convert to K
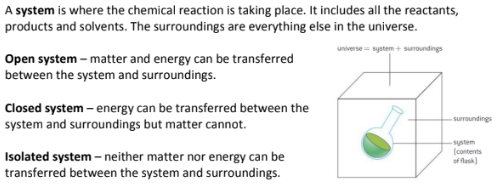
Is bond breaking and bond making endothermic or exothermic? What’s the difference between an open, closed, and isolated system?
Bond Breaking = Exothermic
Bond Breaking = Endothermic
Closed system = energy but not matter can be exchanged with the surroundings
Open system = energy and matter can be exchanged with the surroundings
Isolated system = neither energy nor matter can be exchanged with the surroundings
What is enthalpy change referring to? What is enthalpy change like in exo vs endo thermic reactions?
The heat transferred during a reaction in a system at SATP Standard State (298 K, 100kPA)
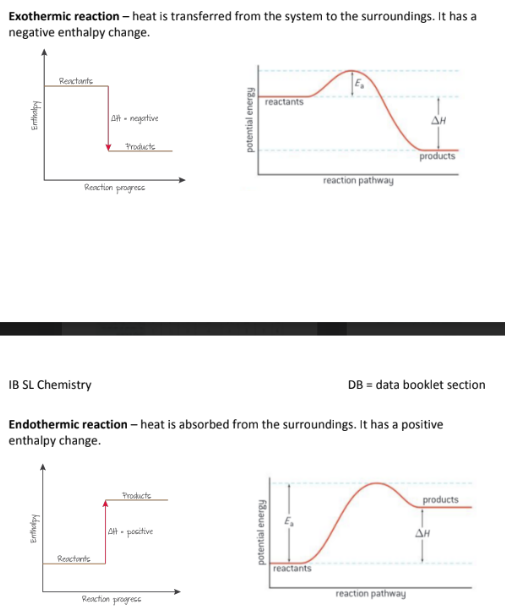
Draw a reaction coordinate diagram for Exothermic and Endothermic Reactions?
What are some of the assumptions that are made when calculating enthalpy change?
It doesn’t take into account heat loss to surroundings, incomplete combustion, or the assumptions we made about specific heat capacity
How do you convert from kJ to J?
1 kJ = 1000 J