Functional groups: classification of organic compounds
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
empirical formula
simplest whole number ratio of atoms in molecule
molecular formula
actual number of atoms in a molecule
structural formula
spatial arrangement of all atoms and bonds in a molecule
condensed:
enough info is shown to make structure clear, but most bonds omitted
only important bonds are shown, e.g double/triple bonds
functional groups are shown using brackets
skeletal formula
all of the carbon-carbon bonds represented by lines
end of each line and point where 2 lines meet = carbon atom
most hydrogen atoms removed except those part of functional group
stereochemical formula
shows relative positions and 3D geometry of atoms and groups of atoms around chiral carbon
standard convention:
bonds in plane of paper are drawn as solid lines
bonds coming forward out of plane towards you drawn as wedge
bonds going backwards out of plane are drawn as dashed wedge
functional groups
groups of atoms found in organic compounds
homologous series
a family of similar compounds, having the same functional group, therefore similar chemical properties
successive members differ by CH2
have the same general formula
have gradually changing physical properities
as homologous group is ascended, size of molecule increases
alkanes
CnH2n+2
FG: alkyl
suffix: -ane
alkenes
CnH2n
FG: alkenyl
suffix: -ene
alkynes
CnH2n–2
FG: alkynyl (C=-C)
suffix: -yne
halogenoalkane
CnH2n+1X
FG: halogeno
prefix: fluoro ..etc
alcohol
CnH2n+1OH
FG: hydroxyl
suffix: -hydroxy, -ol
aldehyde
CnH2nO
FG: carbonyl (C=OH)
suffix: -al
R-COH
ketone
CnH2nO
FG: carbonyl (C=O)
suffix: one
R-CO-R
carboxylic acid
CnH2n+1COOH
FG: carboxyl (C=OOH)
suffix: -oic acid
ether
CnH2n+2O
FG: alkoxy (-O-)
amine
CnH2n+1NH2
FG: amino (NH2)
suffix: -amine
amide
CnH2n+1NO
FG: amido (C=ONH2)
suffix: -amide
ester
CnH2nO2
FG: ester (C=OO)
suffix: -oate
physical trends in homologous series
bpt & mpt increases with increased molecular size
each additional CH2 adds 8 more electrons, increasing strength of London forces
naming alkanes
saturated
alk + ane
alk depends on number of carbons in chain
meth, eth, prop, but, pent, hex, hept, oct, non, dec
if any side chains or functional groups, the position of these groups is indicated by numbering the carbon atoms in longest chain starting at the end that gives the lowest possible numbers in the name
hydrocarbon side chain shown by brackets in structural formula
side chain is named by adding -yl to normal alkane stem
if there are 1+ of the same alkyl side chain, di-, tri-
numbers separated from words by hyphen
if there are more than one type of alkyl side chain, side chains listed in alphabetical order
naming alkenes
unsaturated
named using alk + ene
in straight chain of 4+ carbons, position of C=C double bond must be specified
carbon chains numbered starting with end closest to double bond
lowest numbered carbon atom participating in double bond is indicated just before the -ene
naming alkynes
unsaturated
named using alk + yne
in straight chain of 4+ carbon, position of triple bond must be indicated
carbon chains numbered starting with end closest to triple bond
lowest numbered carbon atom participating in triple bond is indicated just before the -yne
naming halogenoalkanes
named using prefix chloro-, bromo-, iodo- with ending -ane
in straight chain of 3+ carbon atoms, position of halogen atom must be specified
carbon chains numbered starting with end closest to halogen
number of carbon atom attached to halogen is indicated just before the prefix
when multiple functional groups, position and type must be given
naming alcohols
alco + ol
if 2 OH groups present, it’s a diol
in straight chain of 3+ carbon atoms, position of OH must be specified
carbon chains numbered starting with end closest to OH
number of carbon atom attached to OH is indicated just before the suffix
naming aldehydes
if carbonyl group is on the end of a chain then it is an aldehyde and has functional group RCHO
named using alkan + al
no need for numbers as aldehyde will always be on carbon 1
naming ketones
ketones have a minimum of 3 carbons and have general functional group formula RCOR
named using alkan + one
after butanone, carbonyl group can have positional isomers so numbering must be used
naming carboxyl acids
named using alkan + oic acid
no need for numbers as carboxyl group always on carbon 1
isomer
compounds that have the same molecular formula but different arrangement of atoms
structural isomers
same molecular formula, different structural formula
3 types of structural isomerism:
functional group isomerism
positional isomerism
branched chain isomerism
functional group isomerism
when diff functional groups result in the same molecular formula
homologous series that can be functional group isomers of eachother:
alkenes and cycloalkanes
alcohols and ethers
aldehydes and ketones
positional isomerism
differences in position of functional group in each isomer
some organic compounds that can be described as having primary, secondary or tertiary structures will exhibit isomerism (alcohols and halogenoalkanes)
primary, secondary, tertiary relate to number of carbon atoms that the functional group carbon is attached to
branched chain isomerism
same molecular formula, but their longest hydrocarbon chain is not the same
caused by branching, where the longest hydrocarbon is broken into smaller pieces and these smaller pieces are added as side chains
branching can only occur with 4+ carbon chains
isomerism in amines
amines follow a different classification system of primary, seconday, tertiary to alcohols and halogenoalkanes
classification based on number of alkyl groups attached to the nitrogen in the amine
primary: nitrogen attached to 1 other carbon atom (alkyl groups)
secondary: nitrogen attached to 2 other carbon atoms (alkyl groups)
stereoisomerism
have the same structural formulas, but differ in their spatial arrangement
2 types of stereoisomers
conformational
configurational
cis/trans isomers
optical isomerism
conformational isomers
occur in saturated compounds
due to free rotation about a single sigma bond
free rotation allows easy interconversion from one isomer to the other
cis/tans isomers in alkenes
occur in unsaturated compounds,
groups attached to C=C carbons remain fixed in position
due to presence of pi bond, free rotation isn’t possible
cis isomers have 2 functional groups on the same side of double bond (both above or both below)
trans isomers have 2 functional groups on opposite sides of double bond (1 above 1 below)
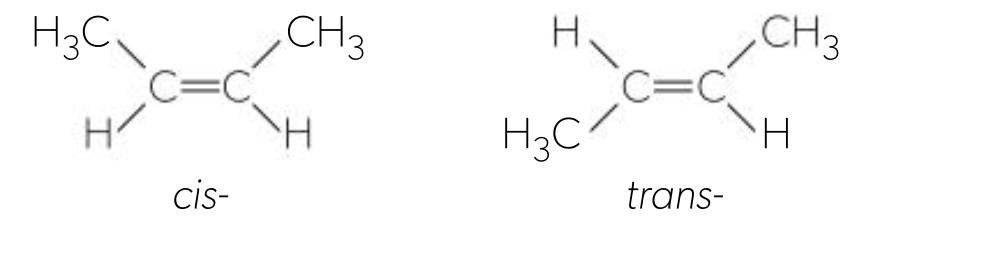
naming cis/trans isomers
for cis/trans to exist, 2 different atoms/groups of atoms on either side of C=C bond are needed
if there is more than one atom/group of atoms on either side of the C=C bond, the naming system fails
works with 3 atoms but 2/3 atoms must be the same and on opposite sides of double bond
cis/trans isomers in cycloalkanes
can also occur in cycloalkanes, as the C-C bond is part of a ring system, restricting rotation
cis isomers occur when atoms are on same side of ring (both above or below)
trans isomers occur when atoms are on opposite sides of the ring (1 above 1 below)
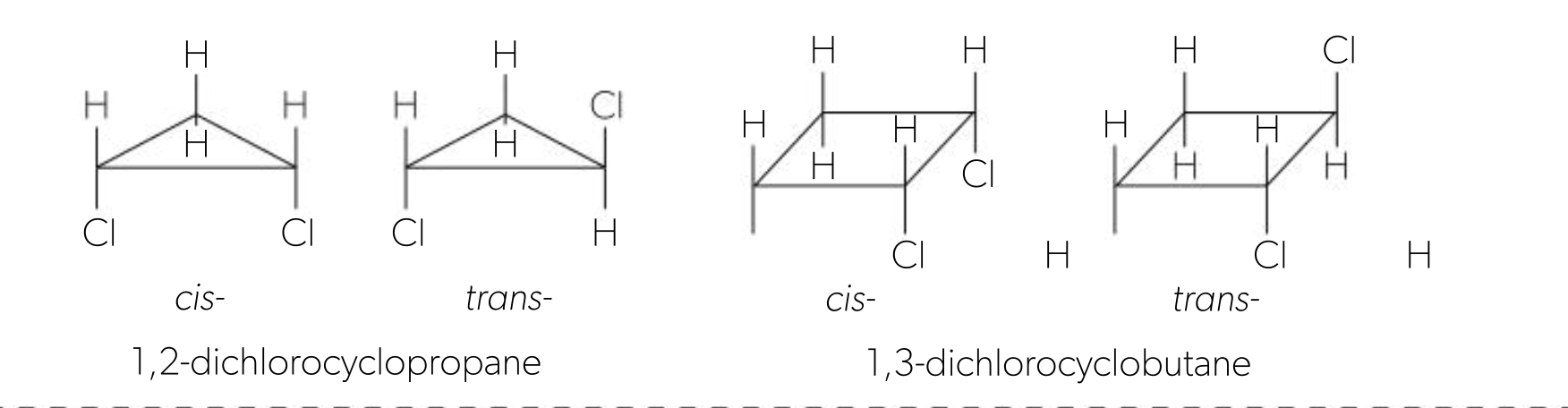
optical isomers
chemicals that contain a chiral carbon
a carbon atom that has 4 different atoms/groups of atoms attached to it
carbon atom is asymmetric
compounds with 1 chiral centre exist as a pair of optical isomers called enantiomers
enantiomers are non-superimposable - mirror images of eachother
diastereomers
compounds that contain more than one chiral centre
are not mirror images of eachother as each chiral carbon has 2 isomers
so have different physical and chemical properties
properties of optical isomers
chemical
different behaviours in chiral environments
physical
identical physical properties except they differ in ability to rotate the plane of polarised light
entantiomers are described as optically active
1 enantiomer rotates plane polarised light in clockwise direction, the other in anticlockwise
rotation of plane polarised light can be used to determine the identity of an optical isomer of a single substance
racemic mixture
a mixture containing equal amounts of each enantiomer
typically optically inactive as the enantiomers will cancel out each other’s effect on plane polarised light
mass spec fragmentation patterns
when compound analysed in mass spec, molecules bombarded with a beam of high speed electrons, knocking off some electrons from molecule forming molecular ions
relative abundance of detected ions form mass spectrum
the peak with the highest m/z value is the molecular ion (M+) peak. the value of m/z is the Mr of the compound
mass spec values for particular fragments in DB
fragmentation patterns
different compounds may have the same Mr, so to determine further, fragments that may appear are analysed as they are characteristic of certain molecules
alcohol fragmentation pattern
tend to lose a water molecule giving rise to peak at 18, below the molecular ion
another common peak found at m/z 31, corresponding to loss of CH2OH+ fragment
IR interpretation
covalent bonds vibrate in different ways, frequency of vibration occurs in IR region of EM spectrum
if organic molecule is irradiated with IR energy that matches the natural vibration frequency of its bonds, it absorbs some of that energy and the amplitude of vibration increases - this is resonance
IR spectroscopy
a technique used to identify compounds based on changes in vibrations of atoms when they absorb IR of certain frequencies
spectrophotometer irradiates sample with IR radiation and detects intensity absorbed
IR only absorbed if molecule has permanent dipole that changes as it vibrates
resonance frequency is the specific frequency at which the bonds will vibrate
IR spectrum shows wavenumbers (reciprocal of wavelength)
characteristic absorptions can be matched to specific bonds in molecules
infrared spectroscopy and GH gases
used to identify pollutants in vehicle emissions in the air
used to measure alcohol levels using roadside breathalysers
IR is passed through breath, characteristic bonds of ethanol measured
proton NMR spectroscopy
only atoms with odd mass numbers show signals on NMR spectra and have property of nuclear spin
in 1H NMR, magnetic field strengths of protons in organic compounds are measured and recorded on a spectrum
samples are irradiated with radio frequency energy, and subjected to strong magnetic field
protons on different parts of a molecule absorb and emit diff radio frequencies
chemical environments in NMR
hydrogen atoms of an organic compound reside in different chemical environments
e.g CH3OH has hydrogen in 2 chemical environments CH3 and OH
protons in the same environment are chemically equivalent
main freatures of H NMR spectra
number of different peaks
each proton in particular chemical environment absorbs at a particular frequency
number of peaks = number of different chemical environments
area under peak
proportional to number of H atoms in that particular chemical environment
each area is integrated and heights of integrated traces can be used to obtain ratio of number of hydrogen atoms in each environment
chemical shift
chemical shift of each absorption is measured in ppm relative to TMS, which has a 0 ppm
splitting pattern
the chemical shift of protons within a molecule is slightly altered by protons bonded to adjacent carbon molecules
spin-spin coupling shows up in high res NMR as splitting patterns
if number of adjacent equivalent protons is n, the signal is split into n+1
TMS as reference standard
tetramethylsilane is used because:
all protons in same environment so gives strong single signal
not toxic and unreactive, so doesn’t interfere w/ sample
volatile, so can be easily removed
peak splitting
high resolution NMR gives more complex signals which appear to be split into sub-peaks - this is multiplicity
splitting pattern of each peak is determined by number of protons in neighbouring environments
if the spin of a neighbouring proton is aligned with the spin of the proton in question, the magnetic field is strengthened, resonance is stronger and chemical shift is higher
if the spin of a neighbouring proton spins against the proton in question, the magnetic field is weakened, resonance is weaker and chemical shift is lower
the resulting high res NMR peak splits into a doublet, 2 equal peaks
when there are 2 neighbouring protons, 3 separate peaks obtained based on effect on magnetic field (stronger, unchanged, weaker), so a triplet is obtained (1:2:1)
when there are 3 neighbouring protons, 4 separate peaks obtained based on effect on magnetic field, so quartet obtain (1:3:3:1)