Cellular Adaptation & Injury
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Cellular adaptation
Cells capabilities to change their structure (size, number & phenotype)
Purpose: to escape & protect from injury
Adapted cell: neither normal/injured
Type of cellular Adaptation
Hyperplasia
Hypertrophy
Atrophy
Metaplasia
Hyperplasia
Increase in size of an organ/tissue caused by increase in number of cells
Cells capable of synthesizing DNA-undergo mitosis
Physiologic :
Hormonal (endometrium/breast/uterus in pregnancy)
Compensatory (partial hepatectomy, erythrocytosis)
Pathologic
Excessive hormone/growth factor stimulation of target tissue
Endometrial hyperplasia (excess oestrogen) - lead to malignancy
Benign prostatic hyperplasia (excess androgens)
Connective tissue cells in wound healing
Mechanism:
Result from growth factor-driven proliferation of mature cells
Increased output of new cells from tissue stem cells
E.g.: after partial hepatectomy, growth factors are produced in liver - active signalling pathways that stimulate cell proliferation

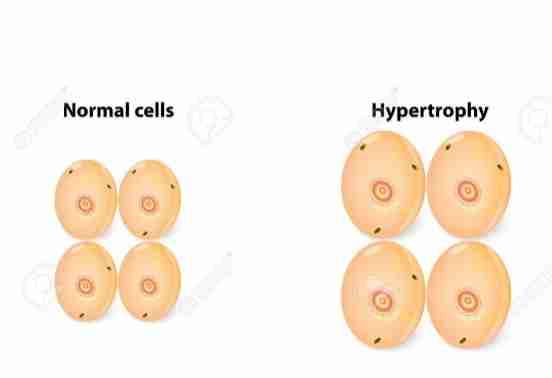
Hypertrophy
Bigger cells
Increase in size of organ/tissue due to increase in size of cell
Physiologic
Hypertrophy of skeletal muscle in muscle builder
Pathologic
Hypertrophy of cardiac heart in hypertension
Mechanism :
Increase in protein synthesis & increase in size/number of intracellular organelles
End result: larger organ
Increase in functional capacity
May occur with hyperplasia
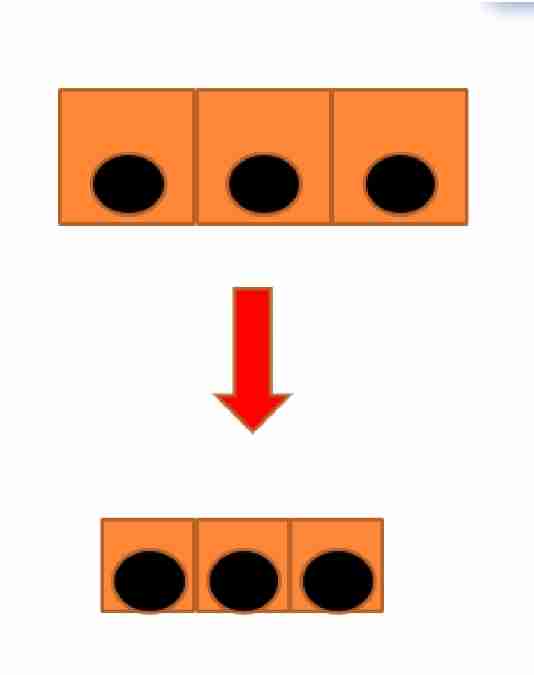
Atrophy
Smaller cells
Atrophy decrease in size of organ/tissue due to decrease in mass of cells
Physiological:
Uterus after delivery
Pathological:
Local, generalised
Denervation
Endocrine stimulation - loss
Aging
Disuse atrophy
Pressure - tumor compression
Ischemia - decrease in blood supply to organ
Inadequate nutrition
Mechanism :
Reduction - cell structural components
Main event is degradation of proteins
2 major system:
Lysosomes
The ubiquitin-proteasome pathway
Atrophic cell - diminished function but NOT DEAD
Metaplasia
Replacement of one differentiated (adult) cell type by other
Fully reversible:
Reprogramming: STEM cell in epthelia/mesenchymal cell in connective tissue
Signals from cytokine, growth factors & Extracellular matrix
Loss of normal function/protection
If persistent, cause malignancy
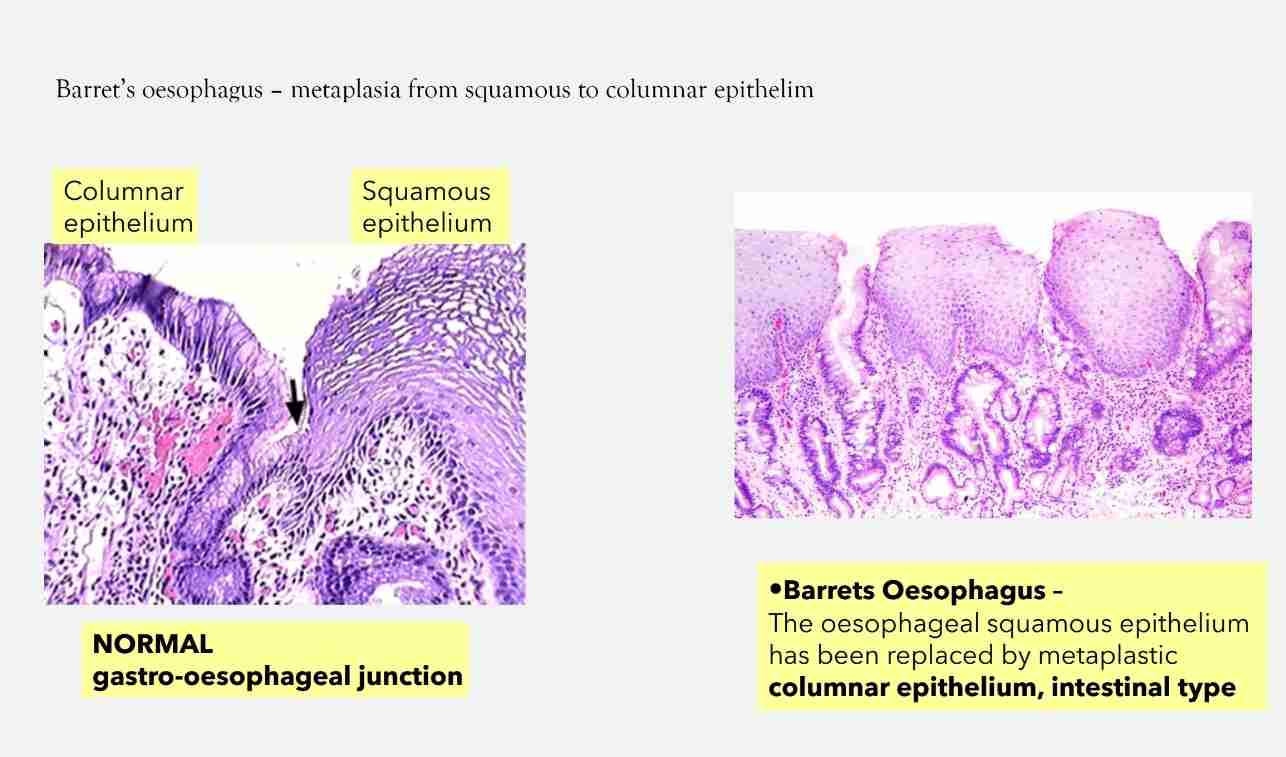
Squamous Metaplasia
Cervix: squamocolumnar junction - replacement of columnar epithelium by squamous epithelium (physiologic)
Occur in respiratory epithelium of bronchus (smoking), endometrium (chronic irritation)& pancreatic ducts (stones)
Associated with long term irritation (smoking) & Vit A deficiency
Often reversible
Causes of cellular injury
Ischemia/hypoxia
Chemical injury : from drugs to poisons
Infectious agents : from viruses to parasites
Immune effector proteins & cells : autoimmune disease
Growth factor stimulation/removal
Nutritient imbalances, specific metabolic inhibitors: protein to vitamin deficiencies, excess cholesterol
Mechanical/physical - trauma, wound, temperature (hot/cold) ionizing radiation
Ischaemic/hypoxic cell injury
Causes - cellular anoxia/hypoxia due to various mechanisms:
Ischemia - obstruction of arterial blood flow, most common
Anaemia - reduction in oxygen carrying RBC
Carbon-monoxide poisoning - altered Hb.
Decreased perfusion of tissues by oxygen carrying blood - cardiac failure, hypotension & shock
Poor oxygenation of blood - pulmunary disease
Mechanism of cell injury
ATP depletion
Mitochondrial damage
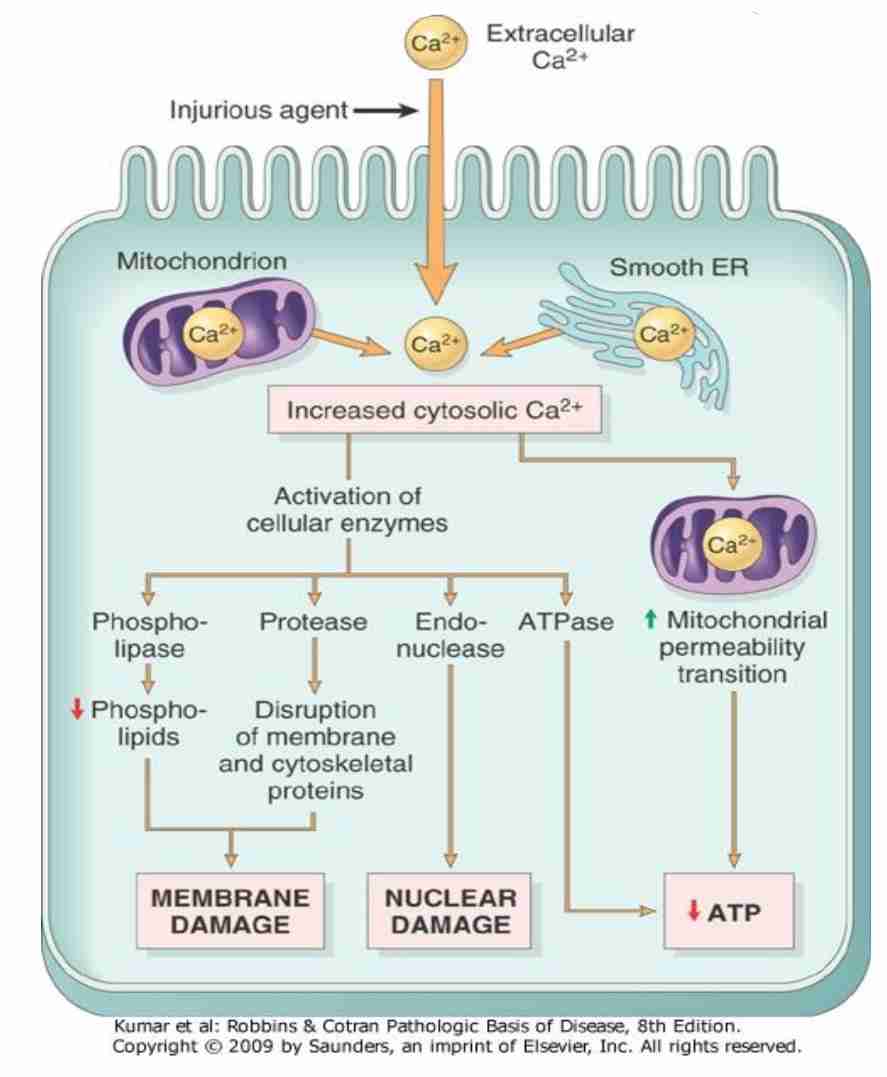
Infux of intracellular calcium & loss of calcium homeostasis
Accumulation of oxygen derived free-radicals (oxidative stress)
Defect in membrane permeability
Damage to DNA & proteins
ATP Depletion
ATP is needed for synthetic & degradative processes in cell
Functional & morphologic consequences of decreased intracellular ATP during cell injury
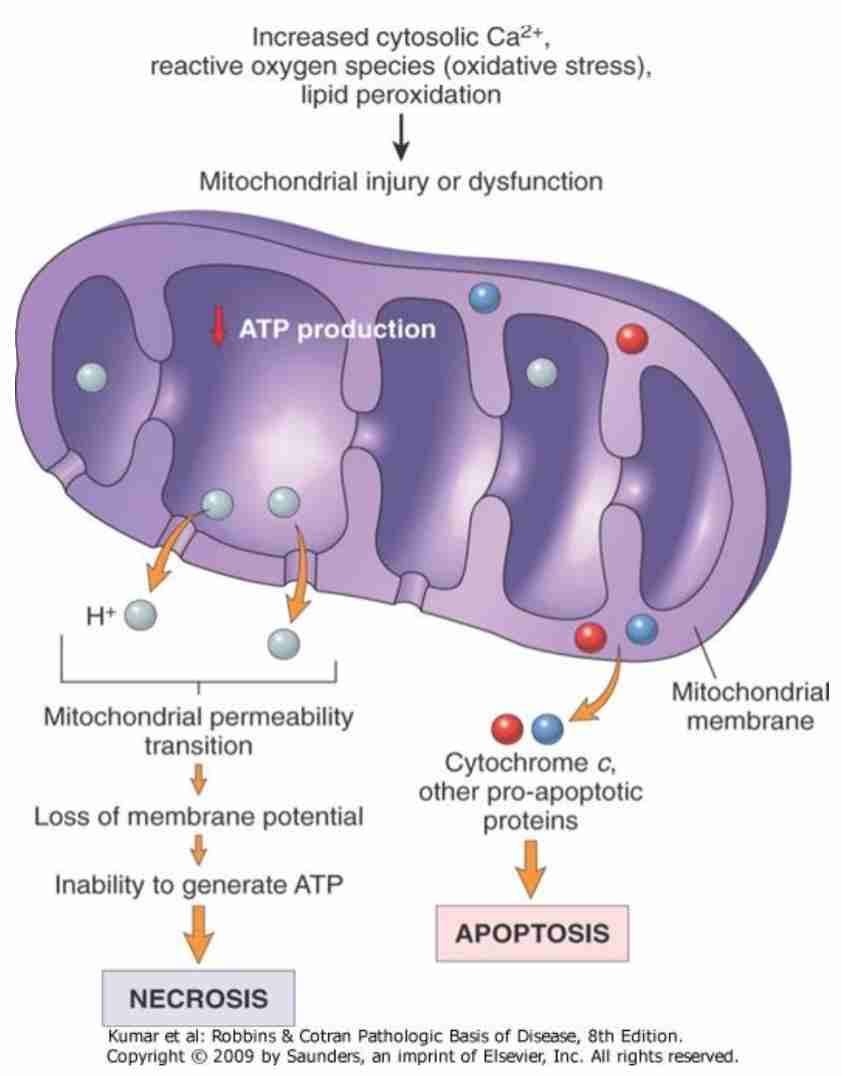
Mitochondrial dysfunction in cell injury
Loss of Calcium homeostasis
Free radical/Reactive oxygen species (ROS) injury
Free radical: molecules with single unpaired electron in outer orbital
E.g.: activated products of oxygen reduction (superoxide & hydroxyl radicals)
Production of ROS increases/scavenging systems are ineffective, result in excess of free radicals - oxidative stress
Mechanism that generates free radicals:
Normal metabolism: aging
Oxygen toxicity: alveolar damage (ARDS)
Ionizing radiation: UV light
Inflammatory process
Drugs & chemicals : introduction of P-450 system
Reperfusion after ischaemic injury
Accumulation of oxygen derived Free-radicals (oxidative stress)
Role of reactive oxygen species in cell injury
O2 is converted to superoxide (O2) by oxidative enzymes in endoplasmic reticulum (ER), mitochondria, plasma membrane, peroxisomes & cytosol
O2 converted to H202 by dismutation & then to OH by Cu2+/Fe2+ (catalyzed Fenton reaction)
H2O2 also derived directly from oxidase in peroxisomes
Another potential injurious radical, singlet oxygen, resultant free radical damage to lipid (peroxidation), proteins & DNA leads to various forms of cell injury
Superoxide catalyzes reduction of Fe3+ to Fe2+, enhancing OH generation by Fenton reaction
Major antioxidant enzymes are superoxide dismutase (SOD), catalase & glutathione peroxidase
GSH reduced glutathione
GSSG oxidized glutathione
NADPH reduced form of nicotinamide adenine dinucleotide phosphate
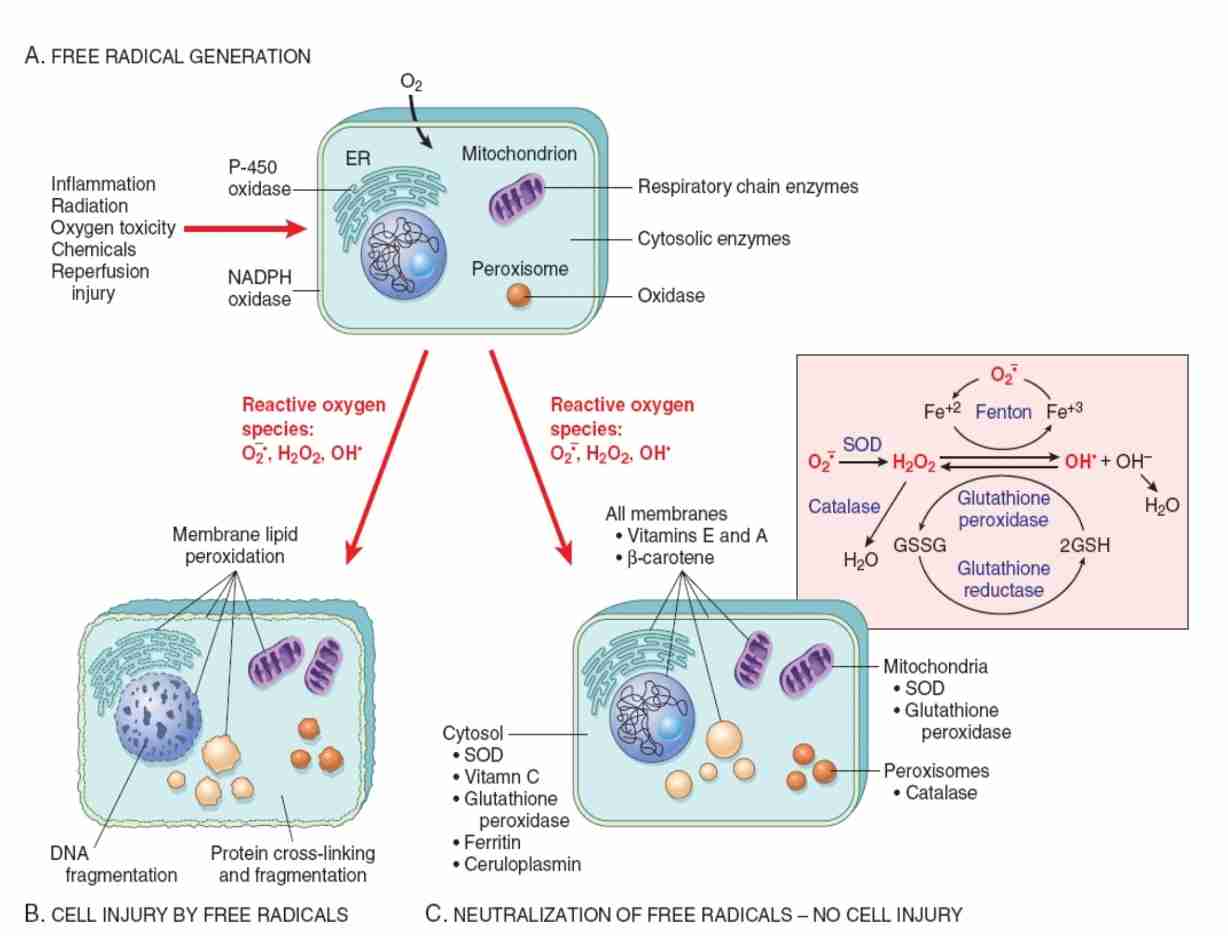
Mechanism of cell injury by ROS
Not adequately neutralized, free radicals can damage cells by 3 basic mechanism:
Lipid peroxidation of membranes:
Double bonds in polyunsaturated membrane lipids are vulnerable to attack by oxygen free radicals
DNA fragmentation
Free radicals react with thymine in nuclear & mitochondrial DNA to produce single strand breaks
Protein cross-linking:
Free radicals promote sulfhydryl-mediated protein cross-linking, resulting in increased degradation/loss of activity
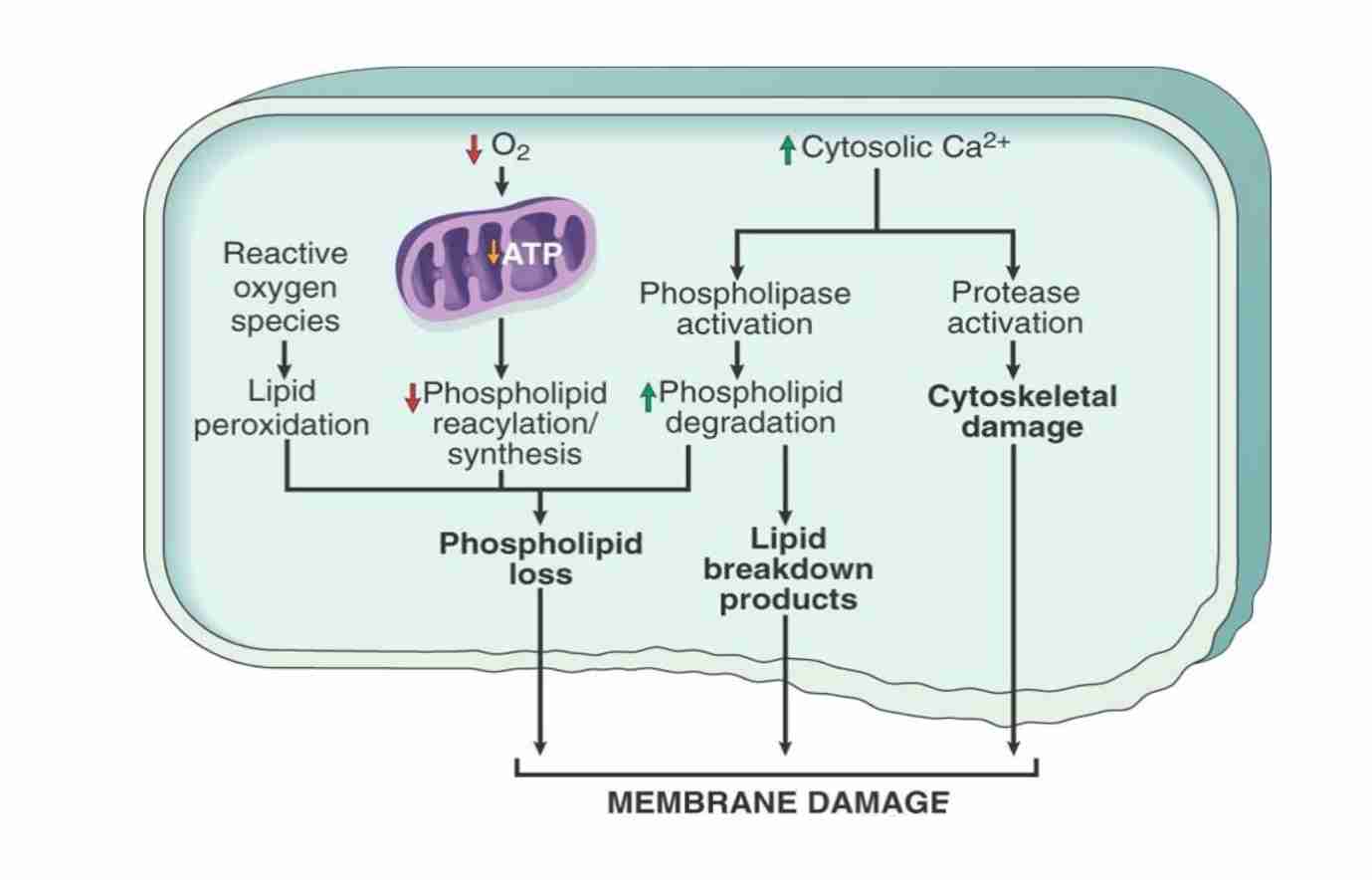
Defect in membrane permeability
Mechanism of membrane damage in cell injury:
Decreased O2 & increased cytoslic Ca2+ are typically seen in ischemia but may accompany other forms of cell injury
Reactive oxygen species, often produced on reperfusion of ischemic tissue cause membrane damage (not shown)
Damage to DNA
Cells have mechanism that repair damage to DNA
If the damage to severe
E.g.: after exposure to DNA damaging drugs, radiation/ oxidative stress
Cell initiates suicide program - death by apoptosis
Cellular response to injury
Ischaemic/hypoxic cell injury
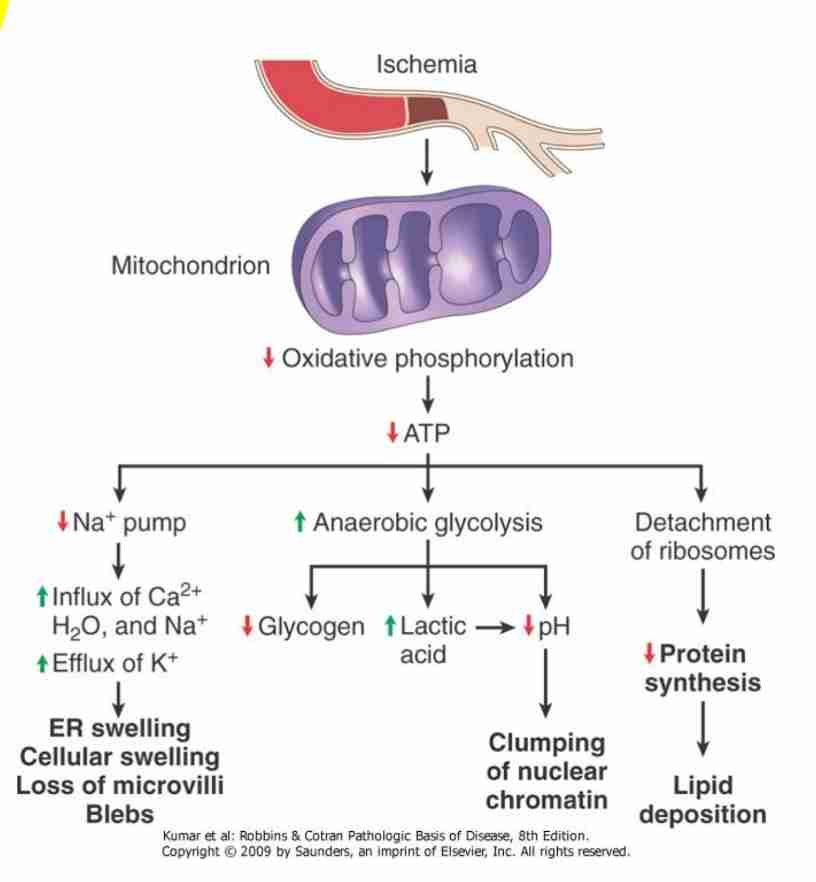
Early stage - first mitochondria is affected leading to decreased oxidative phosphorylation and ATP synthesis
Hypoxic injury becomes irreversible after:
3-5 min for neurons
1-2 hrs for myocardial & liver cells
Many hours for skeletal muscle cells
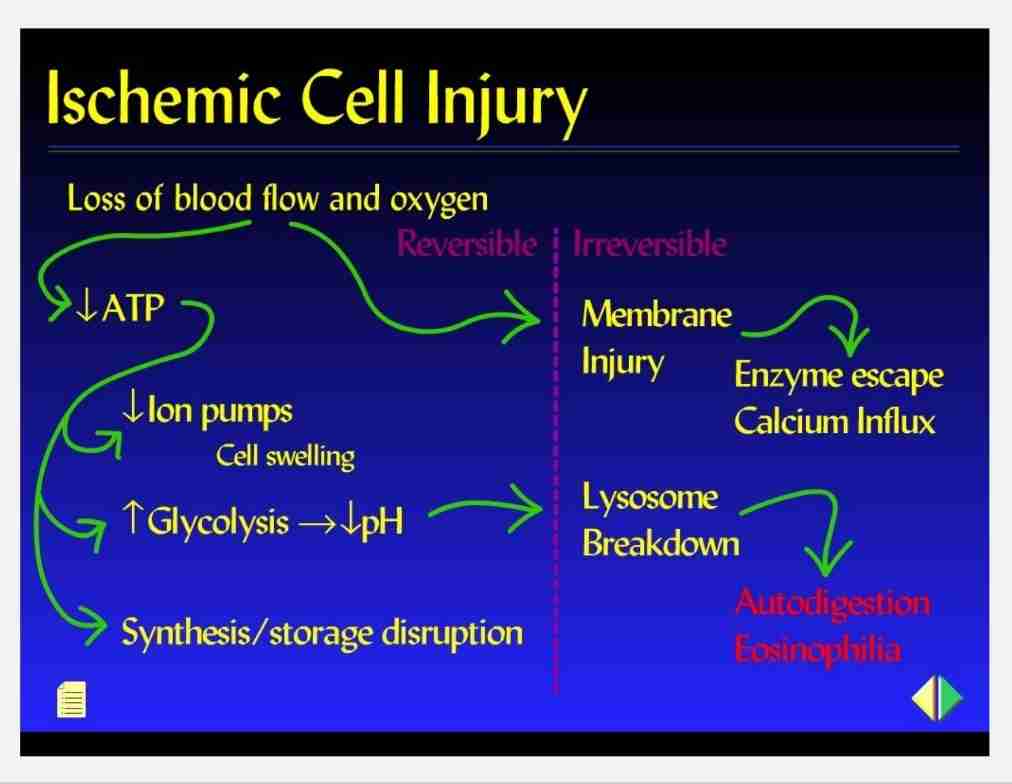
Consequences of decreased ATP availability
Failure of cell membrane pump
Intracellular Na+H20 accumulation, cellular swelling & swelling of organelles : Ca2+ influx
Hydropic change - large vacuoles in cytoplasm
Swelling of endoplasmic reticulum (reversible)
Swelling of mitochondria (reversible to irreversible)
Disaggregation of ribosomes - failure of protein synthesis
Stimulation of phosphofructokinase activity - increased glycolysis, accumulation of lactate & decreased intracellular pH
Acid causes reversible clumping of nuclear material
Late stage
Hypoxic injury causes membrane damage; plasma membrane, lysosomal & organelle with loss of phospholipids
Reversible morphologic signs : myelin figures damage cell membrane & cellular blebbing
Cell death - severe/prolonged injury
Irreversible membrane damage - massive Ca influx, mitochondrial damage & cell death
Release of intracellular enzymes & proteins from necrotic cells into circulation (lab test, indicators of necrosis ; myocardial & liver enzymes)
Vulnerability of cells to hypoxic injury varies - depending on cell type
Ischaemia reperfusion injury
“Reperfusion” Damage
If cells are reversible injured due to ischaemic, restoration of blood flow can paradoxically results in accelerated injury
Clinically important: contribute to myocardial & cerebral infarctions
Exact mechanism: unclear; restoration of flow may expose compromise cells to high concentration of calcium
Reperfusion: increase free radical production from compromised mitochondria & circulating inflammatory cells
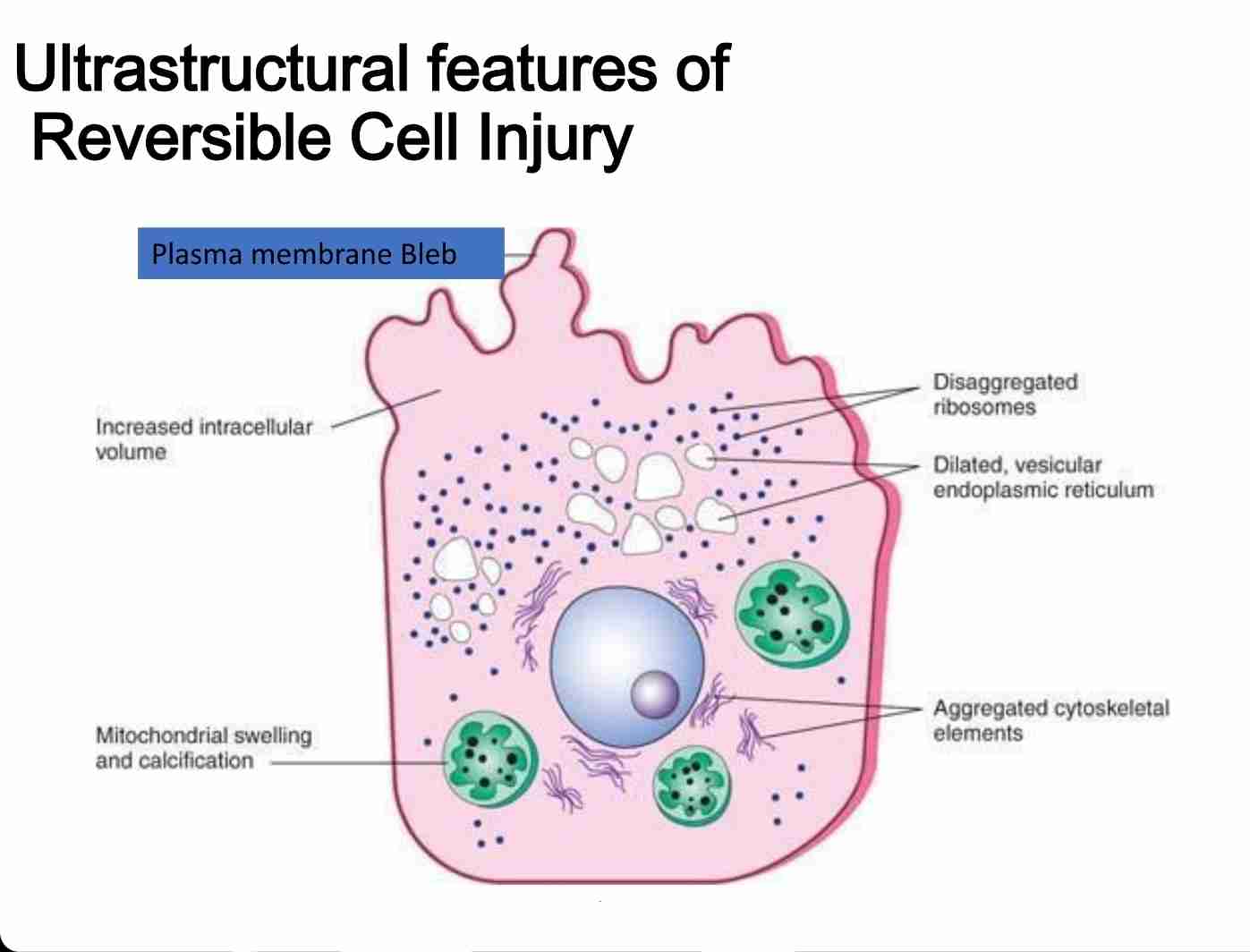
Reversible & irreversible cellular injury
Reversible:
Characterized by generalized swelling of cell and it's organelles
Blebbing of plasma membrane, detachment of ribosomes from endoplasmic reticulum & clumping of nuclear chromatin
Hallmark include:-l
Reduced oxidative phosphorylation with reduced ATP
Cellular swelling caused by changes in ion concentration & water influx
Changes in mitochondria & other organelles
Irreversible :
Characterized by increasing swelling of cell; swelling & disruption of lysosomes, presence of large amorphous densities in swollen mitochondria, disruption of cellular membranes & profund nuclear changes
Latter include nuclear condensation (pyknosis), followed by fragmentation (karyorrhexis)& dissolution of nucleus (karyolysis)
Laminated structure (myelin figures) derived from damaged membranes of organelles & plasma membrane first appear during reversible stage & become more pronounced in irreversible damaged cells
Necrosis & apoptosis
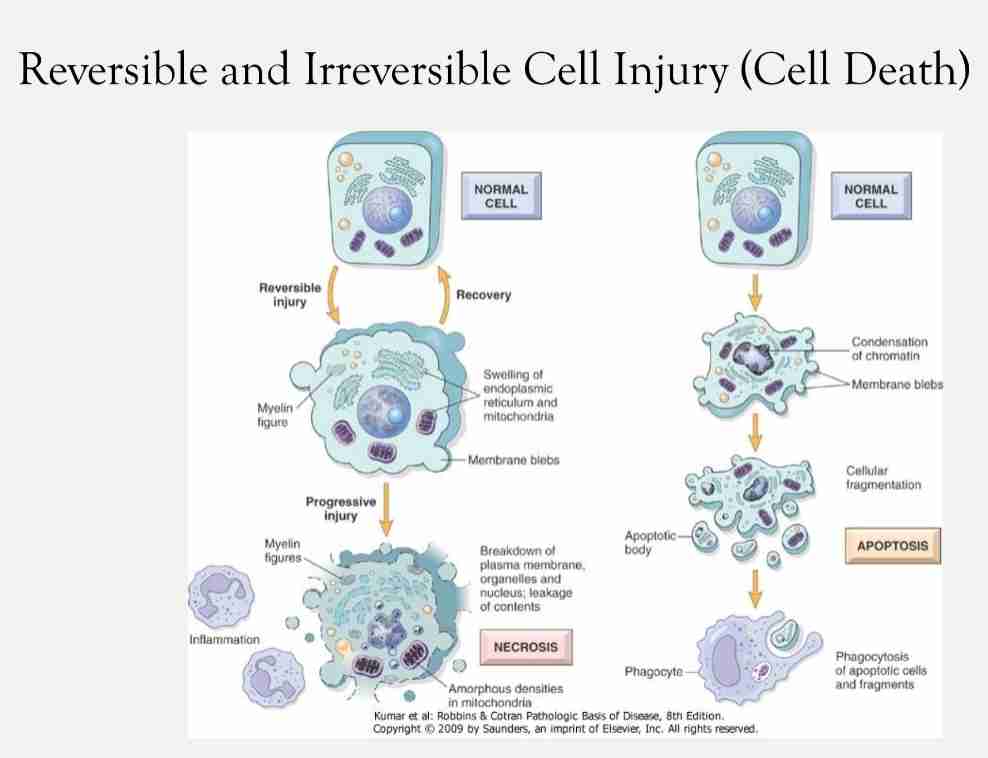
Reversible injury into irreversible injury
With continuing damage, injury becomes irreversible & cells can't recover
Irreversibly injured cell undergoes morphologic change: death cell
2 type of death cell : different in morphology, mechanism, role in disease & physiology
Necrosis: always pathologic process with different morphologic types
Apoptosis
Causes of reversible injury
Decreased ATP levels
Ion imbalances
Decreased pH
Hydropic swelling
Fatty change
Cause of irreversible cellular injury
Severe membrane damage
Influx of extracellular Ca++& release of intracellular Ca++
Lysosomal swelling
Lysosomal rupture
Extensive DNA damage
Mitochondrial vacuolization
Pyknosis, karyolysis/karyorrhexis of nucleus
Mechanism of irreversible cellular injury
Mitochondrial dysfunction
Membrane function disorders - most central factor in pathogenesis of irreversible cell injury
Necrosis
Death of group of cells/tissues in living person
Sequence of morphologic change that follow cell death
Types:
Coagulative necrosis (ischemia)
Liquetactive necrosis (hydrolases)
Fat necrosis (enzymatic/non enzymatic)
Caseous necrosis
Gangrenous (ischemia+ bacterial liquefaction)
Fibrinoid necrosis (immune reactions)
Coagulative necrosis
Cause - ischaemic hypoxic injury
Infraction: tissue death by local lack of oxygen; obstruction of tissue’s blood supply
Coagulation; protein denaturation due to increased acidosis
Seen in most organ : heart, muscle, spleen & gut
Liquetactive necrosis
Seen in brain & bacterial infection
In brain cells:
Brain cells are rich in hydrolytic enzymes & lipids; very little connective tissue
Cells are digested by their own hydrolases
Tissue become soft, lique8, walled off from healthy tissue : forming cysts
In bacterial infection:
Caused by Staphylococcus, streptococcus & etc..
Neutrophils release enzymes to destroy bacteria
Enzymes also destroy tissue causing liquefaction
Fat necrosis
Seen in breast (traumatic fat necrosis) & pancreas (enzymatic fat necrosis)
Traumatic fat necrosis
After trauma to breast, haemorrhage occurs
Cause swelling of breast tissue, with considerable ischaemia & pressure necrosis: necrosis of fat cells
Enzymatic fat necrosis:
Cellular dissolution caused by lipases
Lipases breakdown triglycerides releasing fatty acids - combine with calcium, magnesium & sodium ions making soap: saponification
Necrotic tissue appears opaque and chalky white
Caseous necrosis
Characteristics of tuberculous infection, can involve any organ
Mycobacterium tuberculosis cell wall contains complex wax (peptidoglycolipids) cause toxic effect
Dead cells disintegrated but not completely digested by hydrolases
On gross it has cheese like consistency, soft gray friable
Microscopically there is granuloma/tubercle with central area of caseous necrosis
Gangrenous necrosis
Mostly seen in lower extremities & bowel
Death of tissue due to hypoxia
Dry gangrene: coagulative necrosis without liquefaction skin become dry, wrinkled & dark brown(dusky) in colour
Wet gangrene: complicated by infection & invasion of neutrophils causing liquefaction necrosis
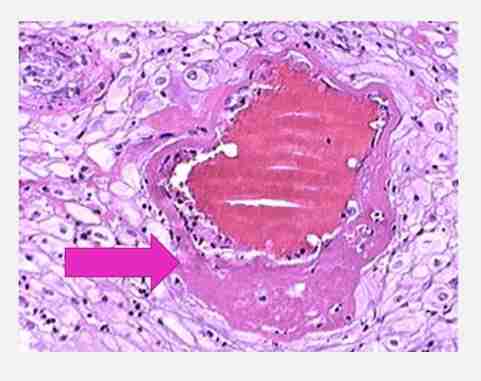
Fibrinoid necrosis
Necrosis associated with immune complex deposition involving blood vessels
Seen when complexes of antigens & antibodies are deposited in walls of arteries
These complexes will activate complacent system & neutrophilic activity
Immune complex deposits caused bright pink amorphous appearance
Apoptosis
Programmed cell death
Important mechanism for removal of cells : cell with irreparable DNA damage(from free radical, viruses, cytoxic immune mechanism)
Protecting against malignant transformation
Occur in normal tissue for regulating number of cell/cell removal during embryogenesis
Physiological Apoptosis
Embryogenesis: formation of digit from limb buds, formation of lumen in gut
Menstrual cycle: endometrial cell loss
Ovulation
Breast: after cessation of lactation
Immune cell development: deletion of immune cells that may react with body’s own tissue
Pathological Apoptosis
Tumors: apoptosis is major cancer killer mechanism. When tumor forms, balance between apoptosis & cell proliferation is disturbed
Viral infection: viral hepatitis, infected hepatocyte can be seen in apoptosis form
AIDS: loss of CD4 T lymphocytes by apoptosis