Industrial Processes
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
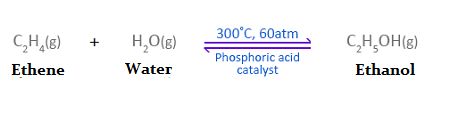
What are the industrial conditions for the production of ethanol?
A pressure of 60-70atm
A temperature of 300°C
A phosphoric acid catalyst
Why is the temperature 300°C?
The reaction is exothermic so lower temperature favour the forward reaction and it means a better yield
However, low temperatures mean a slower rate of reaction so 300°C is a compromise between maximum yield and a faster reaction
Why is the pressure 60-70atm?
Higher pressure favours the forward reaction and increases the yield so 60-70atm is used
Higher pressure also means higher rate of reaction, so ideally you would want an even higher pressure but it’s expensive to produce as you would need stronger pipes and containers
60-70atm is a compromise between max yield and minimum expense
What do you do to save money and raw materials in the production of ethanol?
Only a small proportion of ethene reacted each time
So, the unreacted ethene is separated and recycled back into the reactor
Thanks to this, around 95% of ethene is eventually converted to ethanol
What are the industrial conditions for the production of methanol?
Pressure of 50-100atm
Temperature of 250°C
Catalyst of a mixture of copper, zinc oxide and aluminium oxide
