Immunoglobulin/Antibody (F)
5.0(1)
5.0(1)
New
Card Sorting
1/31
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
1
New cards
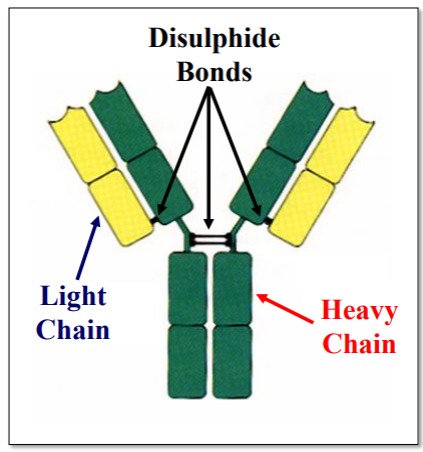
Describe the structure of Immunoglobulin
4 Polypeptide Chains
• 2 Light (L) Chains
• 2 Heavy (H) Chains
Held Together By
Disulphide Linkages
Heavy chains are larger and contain more genetic information.
• 2 Light (L) Chains
• 2 Heavy (H) Chains
Held Together By
Disulphide Linkages
Heavy chains are larger and contain more genetic information.
2
New cards
Describe the variable domains of Immunoglobulin
Found on the N terminal (ends of the Y), Variable regions promote Ag Binding
3 hypervariable regions form loops that bind Ag based on complementarity - Complementarity Determining Region (CDR)
The remaining V domain regions are structurally conserved Framework Regions (FW)
3 hypervariable regions form loops that bind Ag based on complementarity - Complementarity Determining Region (CDR)
The remaining V domain regions are structurally conserved Framework Regions (FW)
3
New cards
Describe the constant domains of Immunoglobulin
Found on C terminal, constant region promotes Effector Functions

4
New cards
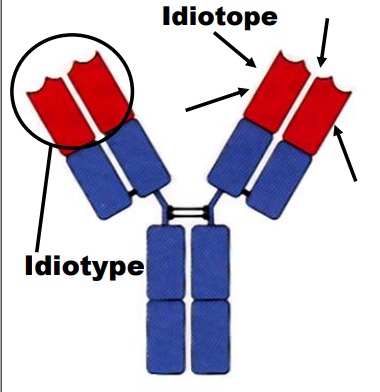
Define: Idiotype
A collection of antigenic determinants contained in the antigen-binding V region. Each individual determinant is known as an idiotype. Immunoglobulin against different antigens have differing idiotypes.
5
New cards
How is antibody activity regulated?
Anti-idiotype antibodies (anti-IgM, anti-IgG etc) bind to the idiotypes of our own antibodies to prevent antigen binding (useful to end Ab activity when an infection is going away)
6
New cards
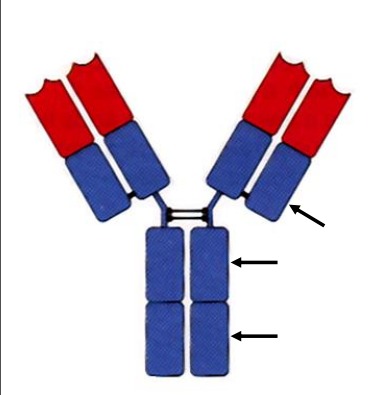
Define: Allotype
Subtle amino acid differences within the same constant region between individuals of the same species. For example IgG1, IgG2, IgG3, etc.
7
New cards
Define: Isotype
5 Classes of heavy chain constant region antigenic determinants: IgA, IgG, IgD, IgM, IgE.
2 Classes of light chain constant region antigenic determinants k, l.
2 Classes of light chain constant region antigenic determinants k, l.
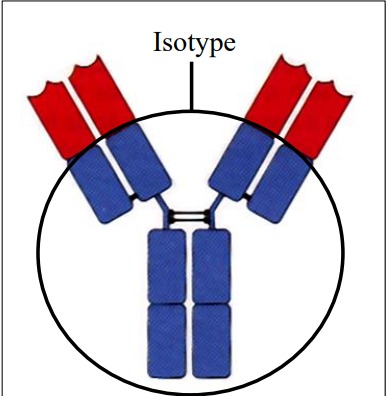
8
New cards
Explain: IgG
- The most abundant immunoglobulin in sera, lymph, cerebrospinal fluid, and peritoneal fluid. 80% of serum immunoglobulin.
- Roles: activates complement, functions as an opsonin by binding to Fc receptors
- 4 subclasses IgG1, IgG2, IgG3, IgG4.
- Roles: activates complement, functions as an opsonin by binding to Fc receptors
- 4 subclasses IgG1, IgG2, IgG3, IgG4.
9
New cards
Define: Opsonin
Extracellular proteins that, when bound to substances or cells, induce phagocytosis.
10
New cards
Rank the IgG subclasses in their ability to activate complement
IgG3>IgG1>IgG2>IgG4
IgG3 is the most flexible, thus the best at activating complement.
IgG3 is the most flexible, thus the best at activating complement.
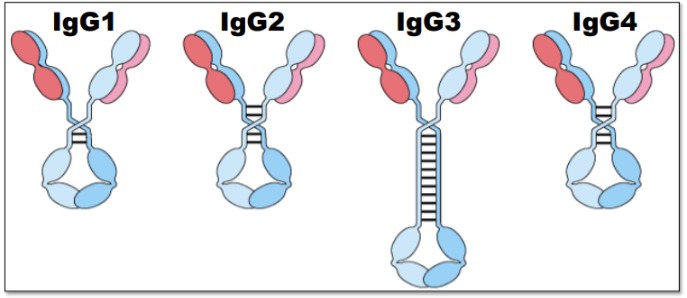
11
New cards
Explain: IgM
- The first immunoglobulin produced
- Makes up 5-10% of serum immunoglobulin (lots is secreted)
- Often found in a pentameric soluble form
- Polyvalent binding agglutinates antigen and activates complement (high avidity)
- J-chain allows for secretion
- Makes up 5-10% of serum immunoglobulin (lots is secreted)
- Often found in a pentameric soluble form
- Polyvalent binding agglutinates antigen and activates complement (high avidity)
- J-chain allows for secretion
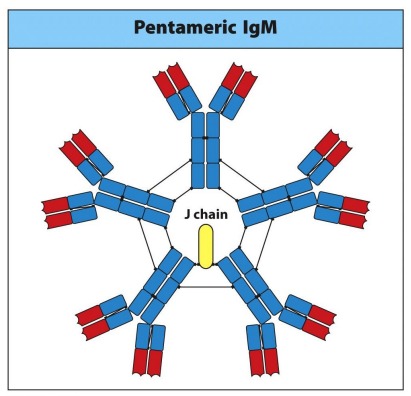
12
New cards
Explain: IgD
- Makes up 0.2% of serum
- Found on the surface of mature B-cells with IgM (may function in the activation of B-cells)
- Function is unknown
- Single hinge is prone to cleavage (breaks down in blood)
- Found on the surface of mature B-cells with IgM (may function in the activation of B-cells)
- Function is unknown
- Single hinge is prone to cleavage (breaks down in blood)
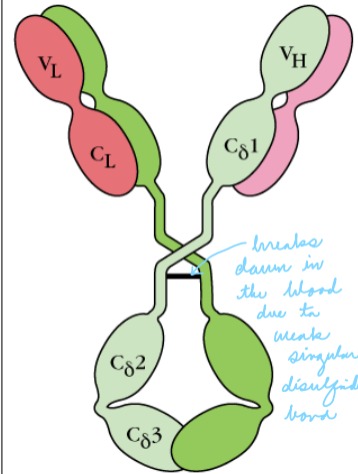
13
New cards
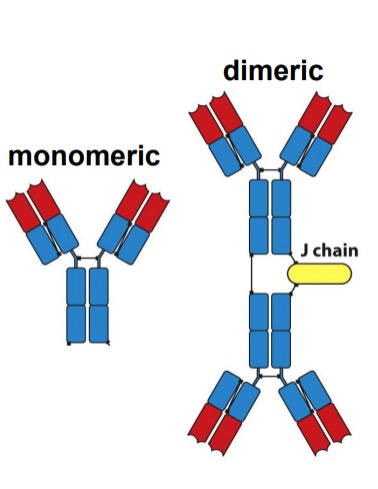
Explain: IgA
- Minor serum immunoglobulin (5-10%)
- The most produced immunoglobulin (75% of total antibody production)
- Most predominant immunoglobulin in secretions
- Serum IgA is monomeric
- Secreted IgA is dimeric (+ J chain) \
- Two subclasses IgA1, IgA2
- The most produced immunoglobulin (75% of total antibody production)
- Most predominant immunoglobulin in secretions
- Serum IgA is monomeric
- Secreted IgA is dimeric (+ J chain) \
- Two subclasses IgA1, IgA2
14
New cards
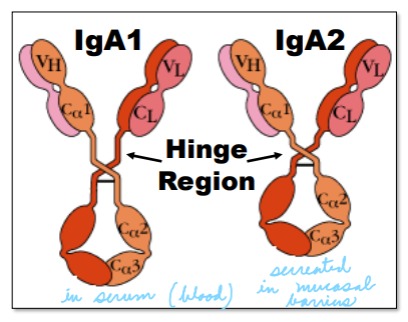
Explain how IgA1 and IgA2 differ
IgA1 is found in serum, 30 amino acids longer (larger hinge), and often cleaved when secreted.
IgA2 is found in secretions, has a shorter hinge, and is thus protected in secretions containing bacteria.
IgA2 is found in secretions, has a shorter hinge, and is thus protected in secretions containing bacteria.
15
New cards
What is required for antibodies to be secreted?
Binding by the J-chain which assists in transcytosis through the epithelial layer into the lumen.
16
New cards
Explain: IgE
- Smallest contribution to serum immunoglobulin
- No hinge region
- Binds to Fc receptors on basophils and mast cells to promote allergic reactions (mediate immediate
hypersensitivity reactions resulting in hay fever,
asthma, hives, anaphylaxis)
- No hinge region
- Binds to Fc receptors on basophils and mast cells to promote allergic reactions (mediate immediate
hypersensitivity reactions resulting in hay fever,
asthma, hives, anaphylaxis)
17
New cards
How can IgE reactions be subdued?
Desensitization
Prolonged exposure to small doses of allergen
1. Could silence cells that produce the recognized allergen
2. Body may stop making IgE and make IgG instead.
Immunotherapy
- Tags all IgE with anti-IgE antibody and promotes phagocytosis (removes all IgE)
Prolonged exposure to small doses of allergen
1. Could silence cells that produce the recognized allergen
2. Body may stop making IgE and make IgG instead.
Immunotherapy
- Tags all IgE with anti-IgE antibody and promotes phagocytosis (removes all IgE)
18
New cards
What do immunoglobulins do?
1) Act as B-cell receptors
2) Activate Complement
3) Neutralize
4) Fc Receptor Binding
2) Activate Complement
3) Neutralize
4) Fc Receptor Binding
19
New cards
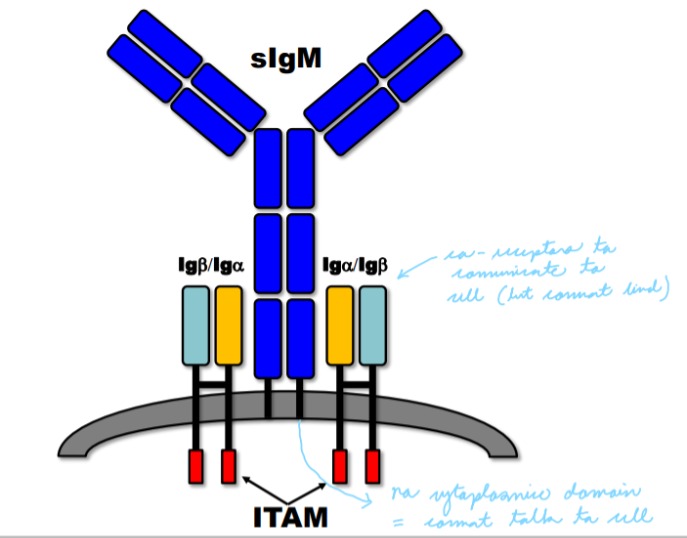
Explain the role of immunoglobulin as B-cell receptors
Surface immunoglobulin (sIg) has short cytoplasmic domains and cannot mediate intracellular signals.
sIG associates with the Ig superfamily members Iga and Igb which generate intracellular signals through Immunoreceptor Tyrosine-based Activation Motifs (ITAM).
sIG associates with the Ig superfamily members Iga and Igb which generate intracellular signals through Immunoreceptor Tyrosine-based Activation Motifs (ITAM).
20
New cards
If surface immunoglobulin bind antigen, but Iga and Igb are not present will the B-cell be activated?
No. To activate the B-cell sIg bind must Ag AND Iga Igb need to mediate the intracellular signal
21
New cards
How to Iga and Igb generate intracellular signals?
Immunoreceptor Tyrosine-based Activation Motifs (ITAM).
22
New cards
Explain the role of immunoglobulin in activating complement
Immunoglobulin bound to antigen can activate the classical pathway of the complement system if the complement component C1q binds to at least 2 Fc regions or 2 antibodies. (1 IgM or 2 IgG - within 40 nm of each other)
23
New cards
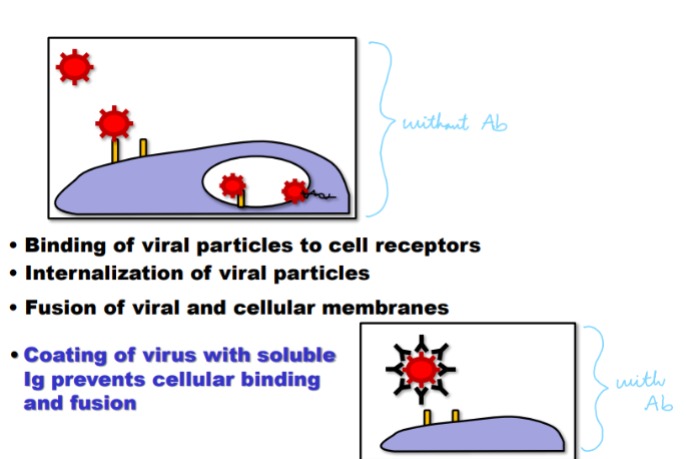
Explain the role of immunoglobulin in neutralization
High affinity Immunoglobulin (IgG, IgA) bound bacteria, viral particles, or toxins prevent their binding to target. Coating bacteria or virus with antibody will inhibit their ability to infect host tissues. Most toxins must bind a cell receptor to mediate detrimental effects → coating toxin
with immunoglobulin protects host cells
with immunoglobulin protects host cells
24
New cards
Explain the role of immunoglobulin in Fc receptors
Several cell surface receptors can bind Ig Fc, Ig binds via its CH2 domain. Ig is bound by the a chain of the FcR. Signaling is mediated through a second molecule, the g chain (Except for FcgRII)
FcR binding can lead to cellular inhibition, activation, phagocytosis, induction of target killing and granule release.
In most cases, the Ig must be bound to antigen to stably associate with the FcR (except for IgE)
FcR binding can lead to cellular inhibition, activation, phagocytosis, induction of target killing and granule release.
In most cases, the Ig must be bound to antigen to stably associate with the FcR (except for IgE)
25
New cards
What are the effects of FcR binding?
Activation of respiratory burst
Inhibition of stimulation
Secretion/release of granules
Degranulation
Induction of killing
Uptake
Ab-dependent cell-mediated cytoxicity
Inhibition of stimulation
Secretion/release of granules
Degranulation
Induction of killing
Uptake
Ab-dependent cell-mediated cytoxicity
26
New cards
Explain: Ab-dependent cell-mediated cytoxicity
A mechanism of cell-mediated immune defense whereby an effector cell of the immune system actively lyses a target cell, whose membrane-surface antigens have been bound by specific antibodies.
1. Coating of target with soluble Ig
2. Binding of NK cell to target via FcgRIII
3. Crosslinking of FcR induces targeted granule release (perforin + granzyme)
4. Death of target cell
1. Coating of target with soluble Ig
2. Binding of NK cell to target via FcgRIII
3. Crosslinking of FcR induces targeted granule release (perforin + granzyme)
4. Death of target cell
27
New cards
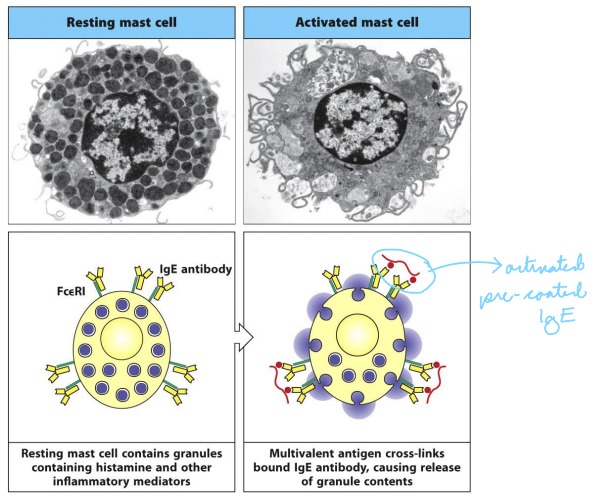
Explain: Granulocyte Degranulation
Mast cells, basophils, and activated eosinophils express a high affinity IgE receptor (FceRI). Soluble IgE stably associates with the FcR in the absence of Ag binding.
Thus these cells are present, pre-coated in polyclonal Ab, ready to engage a variety of Ag.
Crosslinking of the FcR bound IgE by Ag induces
degranulation.Granules release histamine and other pro-inflammatory mediators.
Thus these cells are present, pre-coated in polyclonal Ab, ready to engage a variety of Ag.
Crosslinking of the FcR bound IgE by Ag induces
degranulation.Granules release histamine and other pro-inflammatory mediators.
28
New cards
If the immunoglobulin on B-cells do not bind to antigen what happens?
Conventional B-cells are continuously
produced throughout life. Most IgM+ IgD+ B cells (mature B-cells) that are newly produced have short half-life of 3-4 days. They must encounter antigen to survive, if not they die
produced throughout life. Most IgM+ IgD+ B cells (mature B-cells) that are newly produced have short half-life of 3-4 days. They must encounter antigen to survive, if not they die
29
New cards
What are some hurdles in antibody therapies? How can they be overcome?
Problems:
\- The therapeutic Ab is too antigenic (humans see mouse antibodies as foreign and mount an immune response against the therapeutic antibody)
\- Too short or too long of a half life
\- Poor penetration into tissues
\- Undesired or inefficient effector functions
\
By mutating / engineering the antibody genes in the hybridoma cell one can often reduce/eliminate this issues
\- The therapeutic Ab is too antigenic (humans see mouse antibodies as foreign and mount an immune response against the therapeutic antibody)
\- Too short or too long of a half life
\- Poor penetration into tissues
\- Undesired or inefficient effector functions
\
By mutating / engineering the antibody genes in the hybridoma cell one can often reduce/eliminate this issues
30
New cards
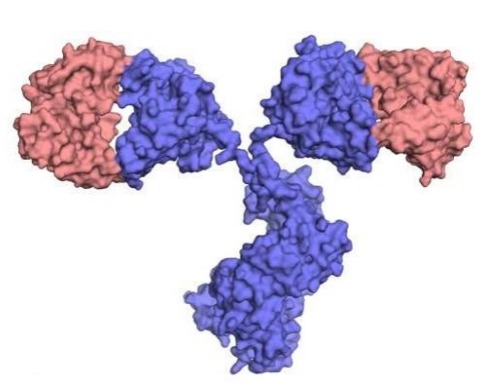
What are chimeric Ab?
“Cloned” VJ and VDJ elements from a mouse Ab fused to the constant domains of a human Ab gene
31
New cards
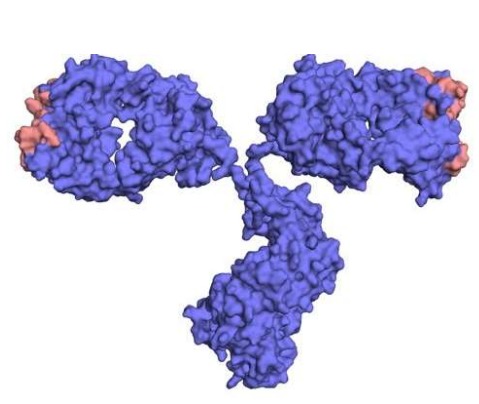
What are humanized Ab?
The specificity of a given Ab is entirely determined by the 6 complementarity determining regions (CDRs) alone, as such the entire variable domains of mouse Ab are not required, just the CDRs.
32
New cards
What are some benefits of antibody therapies?
• Highly specific – less side-effects, collateral damage
• Customizable – can relatively easily retarget the therapy to a different cancer, pathogen or to keep up with evolving “bugs”
• Efficient – less drug needed
• Innovative – bispecific, soluble Fab fragments, antibody-drug conjugates
• Harness the immune system – may replace antibiotics to resolve the problem of multi-drug resistant pathogens
• Customizable – can relatively easily retarget the therapy to a different cancer, pathogen or to keep up with evolving “bugs”
• Efficient – less drug needed
• Innovative – bispecific, soluble Fab fragments, antibody-drug conjugates
• Harness the immune system – may replace antibiotics to resolve the problem of multi-drug resistant pathogens