Cumulative Pharmacy Laws and Regulations
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Pure Food and Drug Act of 1906
This law was passed due to unsanitary conditions and poor labeling; required that the food or drug label could not be false or misleading
This law prohibited interstate commerce of adulterated and misbranded products

Food Drug and Cosmetic Act (FDCA) of 1938
The definitions for food, drugs, dietary supplements and devices were each initially defined under this act for use in humans and other animals
- Required new drugs to be proven only safe before going to market
- Required labeling to have adequate directions for safe use and a list of all ingredients had to be included on the label, and product claims had to be accurate.

Under the Food, Drug, and Cosemetic Act of 1938, what must be included on the label by manufacturers of legend drugs?
Adequate directions for safe use and a list of all ingredients had to be included on the label, and product claims had to be accurate?

Durham-Humphrey Amendment of 1951
Established two classes of drugs: RX drugs and OTC drugs.
Established prescription refills
Required labeling to be different for any drug that was habit-forming or potentially harmful to be dispensed as a prescription drug to a patient with the statement "Caution: Federal law prohibits dispensing without a prescription"
Allowed prescriptions to be taken verbally, rather than in writing
KEFauver-Harris Amendment of 1962
Required new drugs to be proven safe AND EFfective before going to market
Required informed consent from research subjects

True/false - Good manufacturing practices (GMP) requires mandatory compliance from manufacturers, and they are primarily designed to increase a manufacturer's profits
False
Kefauver-Harris Amendment (also known as the Drug Efficacy Amendment) allowed the FDA to set good manufacturing practices (GMP) for the industry and mandated regular inspections of production facilities.

Comprehensive Drug Abuse Prevention and Control Act of 1970
also known as the Controlled Substances Act (CSA)
Enforced by the Drug Enforcement Administration (DEA)
Created five "schedules" of drugs based on their relative potential for abuse
Controlled substances are drugs that have the potential for addiction and abuse and thus require stricter regulation and control.
Established a closed system for manufacturing, distributing and dispensing drugs to reduce drug diversion

The Drug Enforcement Administration (DEA) enforces the CSA, also known as the _______ Act
Comprehensive Drug Abuse Prevention and Control Act of 1970

The _______ enacted the Poison Prevention Packaging Act of 1970 as a requirement for _______, _______, and _______ to be in _______ packaging.
This law required most legend (oral prescription) drugs, many OTC products, and dangerous household chemicals to be packaged in child-resistant (C-R) containers
The Consumer Product Safety Commission enacted this law to prevent accidental poisonings in young children

Under the Poison Prevention Packaging Act , the use of closures that are " reversible " , meaning they are child - resistant in one direction but not in the opposite position are ______.
Permitted only for use in hospice patients
Discouraged , but not prohibited
Permitted only for use in nursing homes
permitted only for use in prescriptions intended for geriatric patients
illegal
Discouraged , but not prohibited
permitted only for use in prescriptions intended for geriatric patients

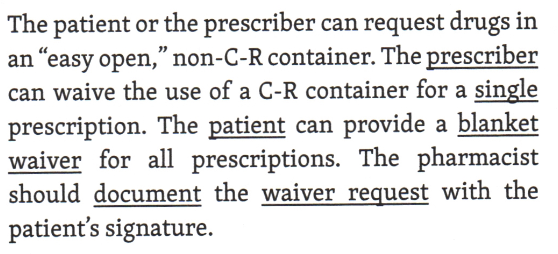
Permission to omit the use of child-resistant closures when dispensing a prescription may be provided by whom _______ or _______.
patient or the prescriber
When should a child-resistant closure be required on a prescription vial (bottle)? Select all that apply.
When a hospital pharmacist dispenses a prescription for a patient who is being discharged home
When a hospital pharmacist fills a prescription for an inpatient
When a community pharmacist dispenses more than ten tablets of a drug
When a hospital pharmacist dispenses a prescription for a patient who is being discharged home
When a community pharmacist dispenses more than ten tablets of a drug
When a hospital pharmacist fills a prescription for an inpatient —> C-R packaging is not required for drugs administered by a healthcare provider.
The Drug Listing Act of 1972 required a ____ on both ____ and ____ drugs.
This law required each individual listed drug to have a unique national drug code (NDC) number that is present on both OTC and prescription drugs

The first segment of a NDC is the _____ code and identifies the _____, _____, or _____ of a drug product.
This number is assigned by the ___.
The 1st segment of a NDC is the LABELER code
It identifies the manufacturer, repackager, or distributor of a drug product.
This number is assigned by the FDA.
Drugs were required to have an individual NDC number according to the Drug Listing Act of 1972.

The second segment of a NDC is the _____ code and identifies the _____, _____, and _____.
This number is assigned by the ___.
The 2nd segment of a NDC is the PRODUCT code
It identifies the strength, dosage and formulation.
This number is determined by the labeler (manufacturer, repackager, or distributor).
Drugs were required to have an individual NDC number according to the Drug Listing Act of 1972.

The third segment of a NDC is the _____ code and identifies the _____ and _____.
This number is assigned by the ___.
The 3rd segment of a NDC is the PACKAGE code
It identifies the package size and type.
This number is determined by the labeler (manufacturer, repackager, and distributor).
Drugs were required to have an individual NDC number according to the Drug Listing Act of 1972.

What requirement did the Medical Device Amendment of 1976 impose on medical devices?
This legislation required medical devices to be classified based on their function
What is the Federal Anti-Tampering Act of 1982?
What are 4 products exempt from this act?
This law made it a federal crime to tamper with OTC products and required tamper-resistant packaging on OTC products.
Exempt products include skin products, insulin, lozenges, and toothpaste.

The Orphan Drug Act of 1983 law provided _____ and __-year _____ for manufacturers who developed drugs to treat rare diseases.
This law provided tax incentives and 7-year exclusivity for manufacturers who developed drugs to treat rare diseases.

Orphan drugs are used to treat rare diseases. (2) What criteria does the drug need to meet in order to be considered an orphan drug?
An orphan drug treats a targeted disease that affects less than 200,000 people in the United States or has no reasonable expectation that the sales revenue will make up for cost of research and development

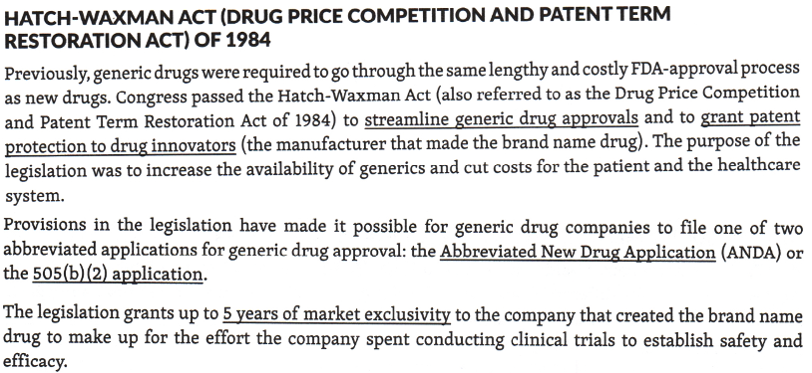
Waxman-Hatch Amendment
also known as the _______
This legislation is also known as the Drug Price Competition and Patent Term Restoration Act of 1984
This law lessened the burden of proof to bioequivalence only for generic manufacturers
Required generic manufacturers to provide bioequivalence to the innovator product before going to market
Allows manufacturers to submit Abbreviated New Drug Application (ANDAs) to market generic drugs
Prescription Drug Marketing Act (PDMA) of 1987
Required states to license prescription drug wholesalers
Specifically limits the re-importation of drug products that were previously exported by a pharmaceutical manufacturer
Prohibits sale of drug samples by pharmacies and coupons nor the resale of drugs by hospitals
Ensure distribution of pharmaceuticals were safe and effective.

A pharmaceutical sales representative may distribute samples of a prescription drug upon the request of the _________.
community pharmacist
physician
medical assistant working for the physician
patient
hospital pharmacist
A pharmaceutical sales representative may distribute samples of a prescription drug upon the request of the physician.
Provisions of the Prescription Drug Marketing Act (PDMA):
Prohibits reimportation of prescription drugs manufactured in the U.S. except by the manufacturer.
Restricts the sale of prescription drug products and samples.
Prescription drug samples can only be distributed if requested by a licensed prescriber.
Establishes permissible distribution channels for drug samples with required record maintenance.
Requires wholesale distributors to be state-licensed and meet uniform standards.

A pharmacist has been filling prescriptions for 35 years. She developed arthritis in her fingers and began to appreciate that it could be difficult for some elderly patients to open safety caps on prescription containers. The pharmacist decided to put all chronic maintenance medications (e.g. hypertension, cholesterol, heart medicines) for seniors into containers that open easily and are not child resistant. Choose the correct statement/s concerning prescription packaging.
The pharmacist can dispense prescriptions in a container that is not child resistant if the patient is over 65, no waiver is needer
All prescription drugs with a few exceptions, must be dispensed in child resistant containers.
The pharmacist can dispense prescription in a container that is not child resistant if the patient signs a waiver.
All prescription drugs with a few exceptions, must be dispensed in child resistant containers.
The pharmacist can dispense prescription in a container that is not child resistant if the patient signs a waiver.

All legend drugs and controlled substances must be packaged in child - resistant containers. Which of the following are considered "exceptions to the rule" ?
Select all that apply .
anhydrous cholestyramine
sodium fluoride products containing less than 264 mg of NaFl per package
sublingual isosorbide dinitrate in strengths > 10 mg
chewable amoxicillin
sublingual nitroglycerin
anhydrous cholestyramine
sodium fluoride products containing less than 264 mg of NaFl per package
sublingual nitroglycerin
According to the _____, this law restricted patients from buying drugs in other countries and bringing them into the U.S., except under two conditions:
Prescription Drug Marketing Act (PDMA) of 1987
The quantity is 90-day supply, and is for the patient (cannot be resold).
An effective treatment is not available in the U.S., the condition is serious, and the drug being imported has no unreasonable risk

Reimportation of prescription drugs from another country is illegal, generally speaking.
However, under a compassionate use policy , the FDA permits personal importation of small amounts of drugs if they are not approved in the US and if they are used for the treatment of serious conditions for which there is no satisfactory treatment available in the US .
Individuals transporting a non - controlled drug on their person into the US may do so as long as the quantity does not exceed a ___ day supply .
90 days

(3) Omnibus Budget Reconciliation Act of 1990 (OBRA ’90)
Required RPhs to conduct prospective DURs and counsel all Medicaid patients prior to dispensing a new prescription or refill.
This law required each state to have a drug utilization review (DUR) to receive reimbursement for services provided to Medicaid patients.
Intended to save the federal government money by improving therapeutic outcomes

True/False - OBRA ’90 required pharmacists to counsel all patients.
FALSE!
OBRA requires each state to have prospective drug utilization reviews (DUR) and counseling for all Medicaid beneficiaries performed by RPhs before dispensing a new prescription or refill in order to receive reimbursement for services provided to Medicaid patients.
(2)Dietary Supplement Health and Education Act (DSHEA) of 1994
established regulations for dietary supplements that were different from those for drugs or conventional foods
requires manufacturers claims about vitamins and minerals to be true

The Food and Drug Administration Modernization Act (FDAMA) of 1997 did the following:
- updated the labeling requirement, "Caution: Federal law prohibits dispensing without a prescription" to be replaced with “______”
- created a _____________ for high-priority drugs
The Food and Drug Administration Modernization Act (FDAMA) of 1997
- updated the labeling requirement, "Caution: Federal law prohibits dispensing without a prescription" , has been replaced with “RX only“.
- created a fast-track process/approval process for high-priority drugs

In 2010, what did the Affordable Care Act (ACA) require for every individual?
The Affordable Care Act (ACA) of 2010 required all individuals to have health insurance

The Drug Quality and Security Act (DQSA) of 2013 differentiated between ______ (____) and ______ (____) and gave oversight of large-scale ____ facilities to which agency?
The Drug Quality and Security Act (DQSA) of 2013 differentiated between compounding pharmacies (503A) and outsourcing facilities (503B) and gave oversight of large-scale compounding facilities to the FDA.

What did the Medicare Modernization Act of 2003 create?
created four unique Medicare programs
The Combat Methamphetamine Epidemic Act of 2005 established ________ limits and ________ requirements for which drug?
This legislation established sales limits and record-keeping requirements for pseudoephedrine
True/False - The Medicaid Tamper-Resistant Law of 2007 required all electronic prescriptions for Medicaid patients.
False
Enforcement of the Poison Prevention Protection Packaging Act falls under the jurisdiction of ____
Consumer Product Safety Commision

When refilling a prescription that requires a poison prevention container component , the pharmacist must always replace ____.
Select all that apply.
the plastic container / vials
the plastic lid closure
the glass container
the plastic container / vials
the plastic lid closure

Which of the following types of drug products are required to be dispensed in poison prevention packaging? Select all that apply.
A bronchodilator in a metered aerosol container
An analgesic for a child
An analgesic for an adult
Estrogen tablets for a female over the age of 65
An analgesic for a child
An analgesic for an adult
Estrogen tablets for a female over the age of 65 —> oral contraceptives in mnemonic packages (like birth control packets), hormone replacement therapy products, and conjugated estrogens are exempt from child-resistant packaging requirements.
However, estrogen tablets not in mnemonic packages and is dispensed as a oral prescription do require child-resistant packaging as they pose a risk if ingested by children.
The PPPA required the use of child-resistant (C-R) containers for many OTC products, most oral prescription drugs and dangerous household chemicals.

Under the Kefauver-Harris Amendment of 1962, the ______ was given oversight to regulate the advertising of prescription drugs, while the ______ continues to regulate advertising of OTC drugs.
Under the Kefauver-Harris Amendment of 1962, the FDA was given oversight to regulate the advertising of prescription drugs, while the FTC continues to regulate advertising of OTC drugs.

Prescription refills were established under which amendment?
Durham-Humphrey Amendment of 1951

In no situation should a pharmacist obstruct a patient's legal right to receive a lawful medication. In the case Noesen v . State ( WI ) , a pharmacist was found guilty of unprofessional conduct because the pharmacist failed to transfer a prescription from one pharmacy to another based on conscientious objection.
The pharmacists's failure transfer the prescription was deemed a danger to the health and safety of the patient and substantially departed from the standard of care of a pharmacist . What should you do if you have a conscientious objection to dispensing a medication for a patient ?
Explain your conscientious objection to the patient
Transfer the prescription to another pharmacy that you've identified and transfer the prescription
Provide your conscientious objection in writing to your employer in advance ( if possible)
If another pharmacist is on duty, ask the other pharmacist if they are willing to fill the prescription
ALL OF THE ABOVE
Explain your conscientious objection to the patient
Transfer the prescription to another pharmacy that you've identified and transfer the prescription
Provide your conscientious objection in writing to your employer in advance ( if possible)
If another pharmacist is on duty, ask the other pharmacist if they are willing to fill the prescription

A drug company is marketing an OTC drug, ColdImmune Health Formula, as a product that will prevent colds. Which regulatory body is responsible for enforcing the accuracy of this claim?
Federal Trade Commission
Under the Kefauver-Harris Amendment of 1962, the FDA was given oversight to regulate the advertising of prescription drugs, while the FTC continues to regulate advertising of OTC drugs
The FDA is responsible for approving prescription drugs before a drug is available on the market. When must a new drug application (NDA) be submitted to the FDA?
When a drug manufacturer develops a new drug and requires FDA approval
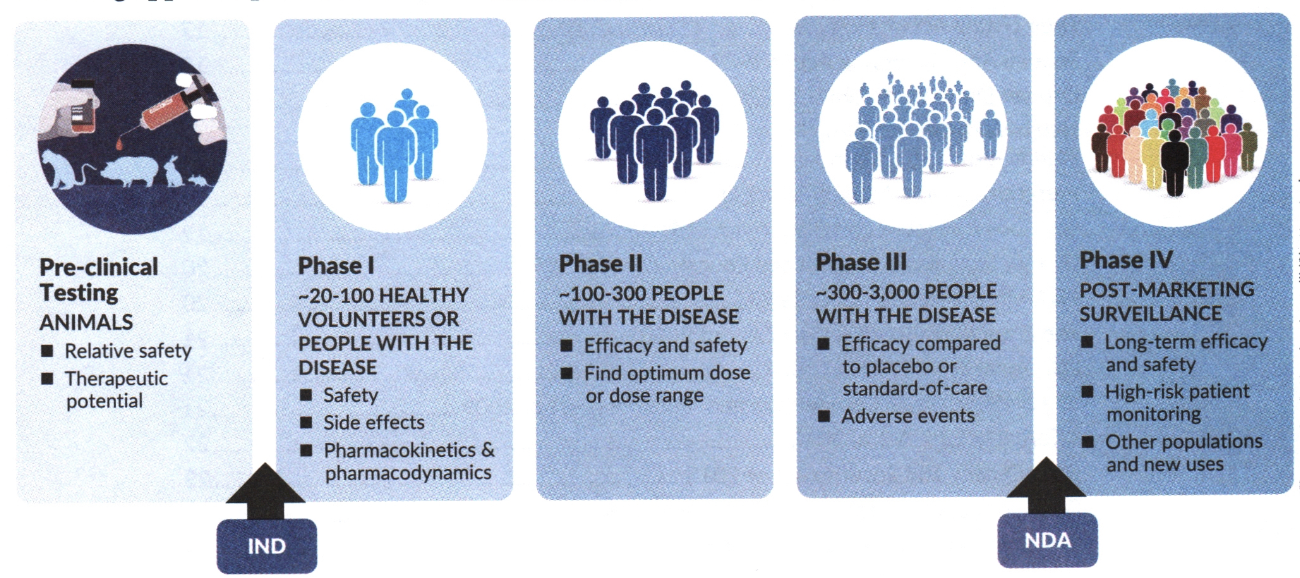
List four changes accepted under a supplemental new drug application (sNDA) or a supplemental biologics license application (sBLA).
Change the label
expanding the approved indications (new uses) of the drug
Modifying the dosage/strength
Changing the manufacturing process

A pharmaceutical company would like to market a new dosage for a drug. Which type of application must the pharmaceutical company submit to the Food and Drug Administration?
supplemental new drug application (sNDA)

EyeMerica drug company would like to make a generic version of a prescription drug product with an expired exclusivity period.
Which type of application must EyeMerica submit to the Food and Drug Administration?
Abbreviated New Drug Application (ANDA)
The Hatch-Waxman Act (also referred to as the Drug Price Competition and Patent Term Restoration Act of 1984 granted patent protection to drug innovators and streamlined generic drug approvals in which generic drug companies only file one of two abbreviated applications for generic drug approval: the Abbreviated New Drug Application (ANDA) or the 505(b)(2) application

A pharmaceutical company is developing a new biological drug to treat Alzheimer's Disease. Which type of application must be the pharmaceutical company submit to the Food and Drug Administration?
biologics license application (BLA) is used for FDA approval of biologics
