CHEM 40A - Bertrand
1/79
Earn XP
Description and Tags
Functional groups, biomolecules, ch9 phosphate stuff, other vocab terms
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
1-carbon chain
meth-
2-carbon chain
eth-
3-carbon chain
prop-
4-carbon chain
but-
7-carbon chain
hept-
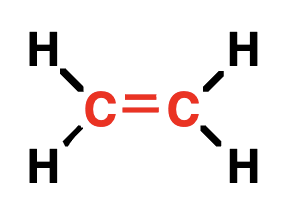
alkene
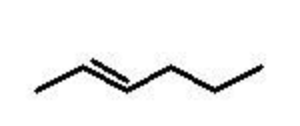
alkene
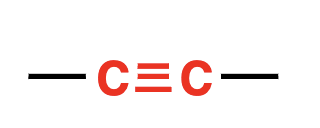
alkyne
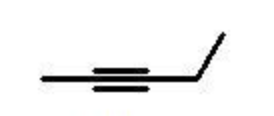
alkyne
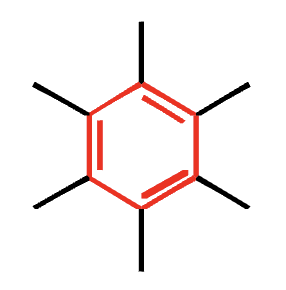
arene
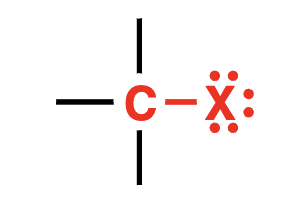
X = any halide
alkyl halide
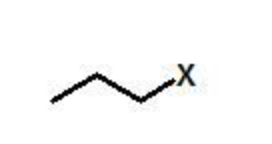
X = any halide
alkyl halide
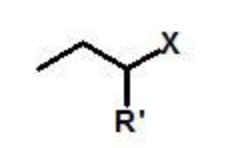
X = any halide
alkyl halide
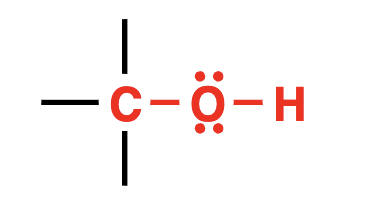
alcohol
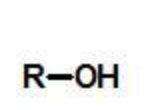
alcohol
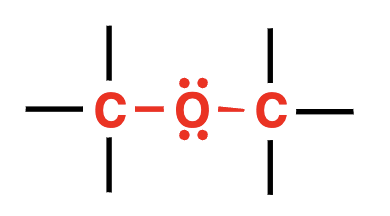
ether
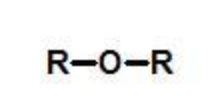
ether
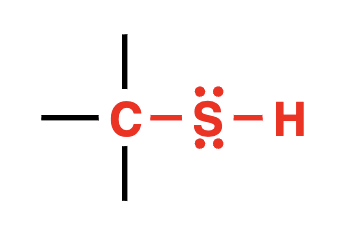
thiol
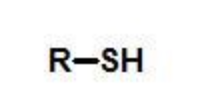
thiol
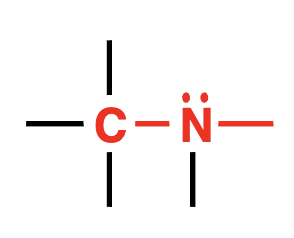
amine
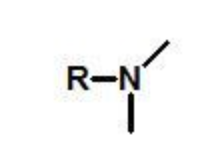
amine
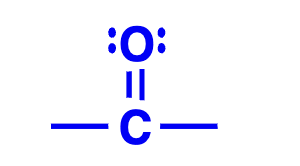
carbonyl group
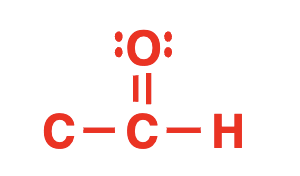
aldehyde
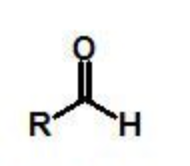
aldehyde
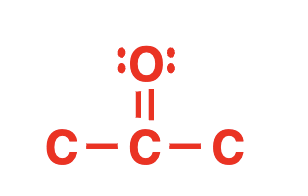
ketone
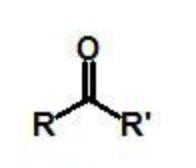
ketone
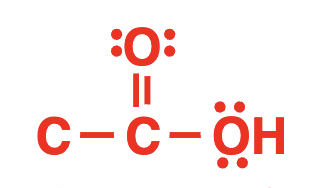
carboxylic acid
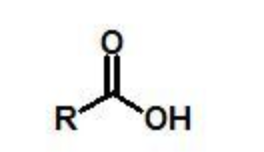
carboxylic acid
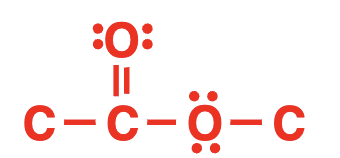
ester
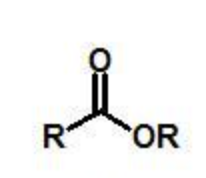
ester

amide
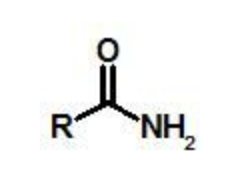
amide
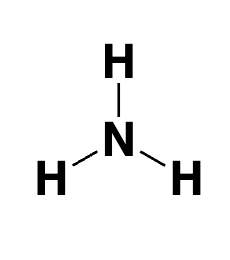
ammonia
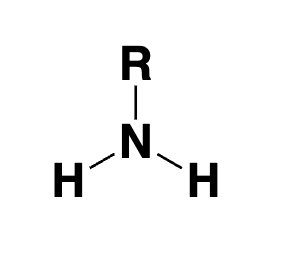
primary amine
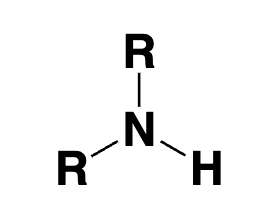
secondary amine
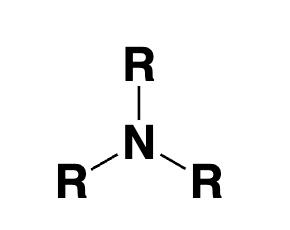
tertiary amine
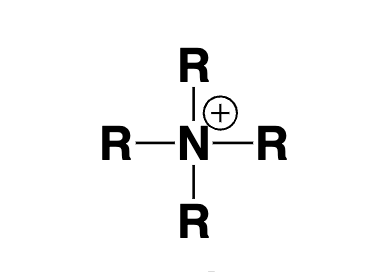
quaternary ammonium ion
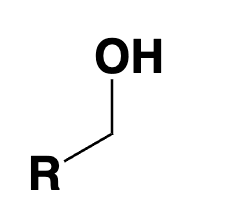
primary alcohol
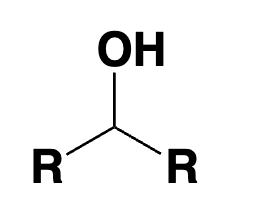
secondary alcohol
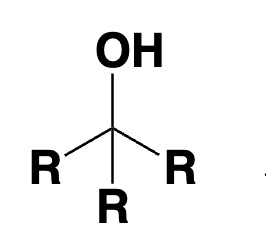
tertiary alcohol
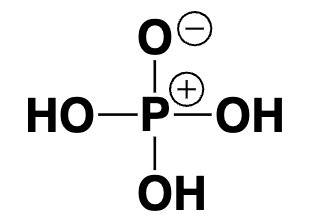
phosphoric acid

phosphoric acid
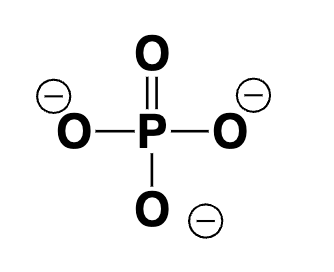
phosphate (-3 charge)
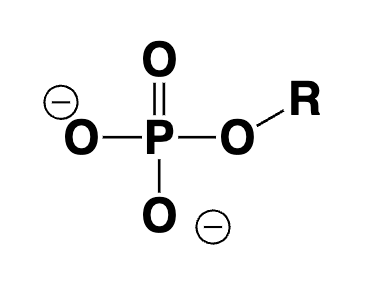
phosphate ester
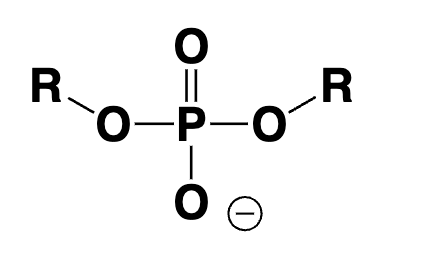
phosphate diester
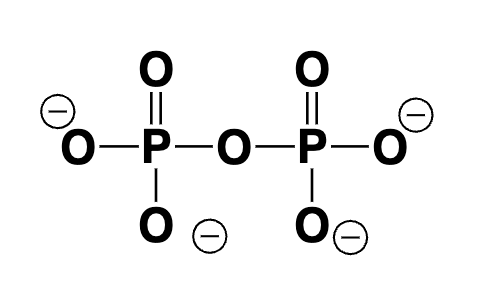
phosphate anhydride
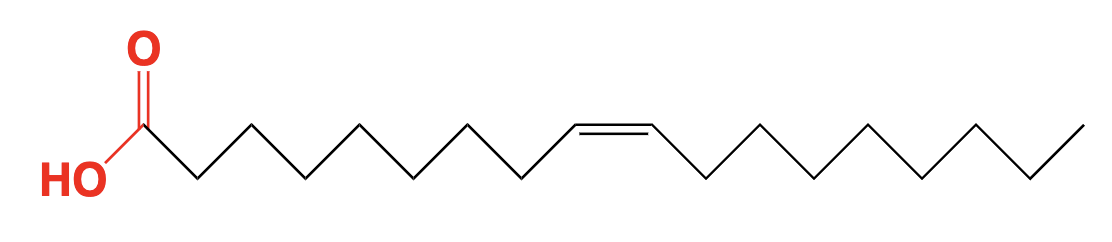
lipid (fatty acid) - hydrocarbon chain with carboxylic acid at the end
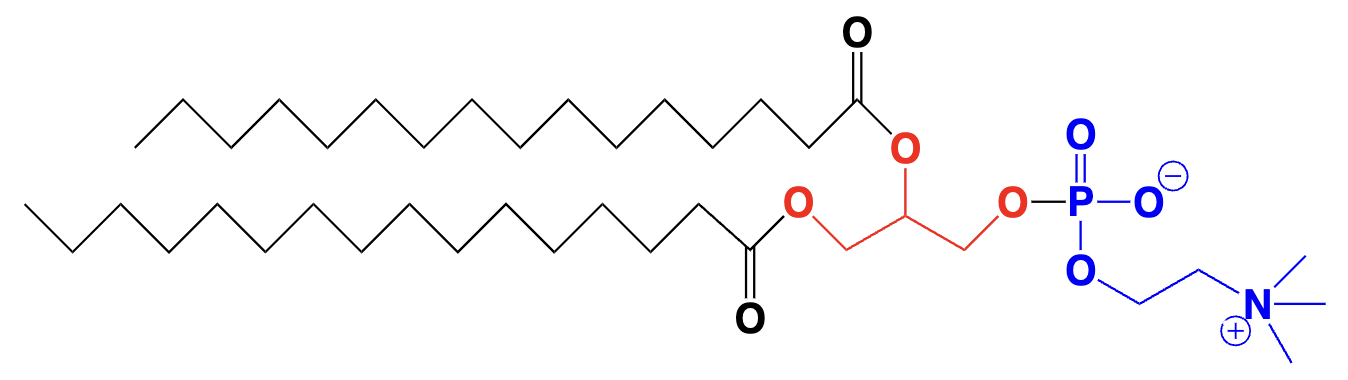
lipid (fat/ oil) - fatty acids linked by an ester group
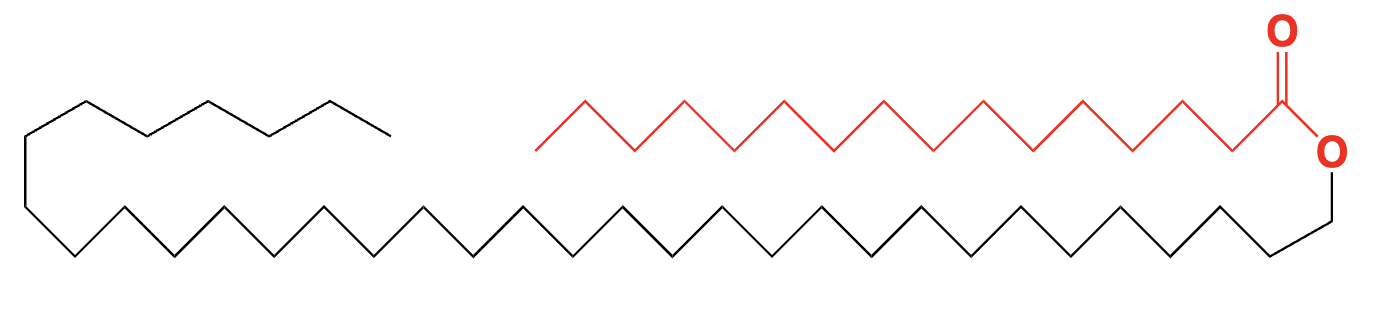
lipid (wax) - fatty acid linked to long chain alcohols through ester group
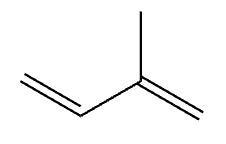
isoprene, found in lipids (isopropenoids)
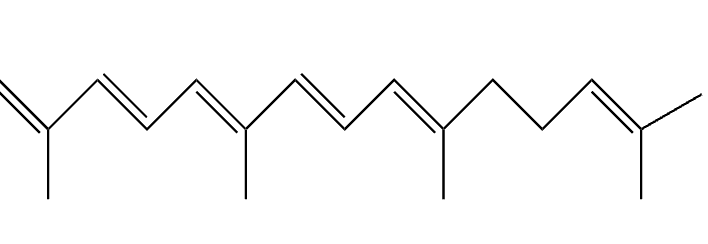
lipid (ispropenoid)
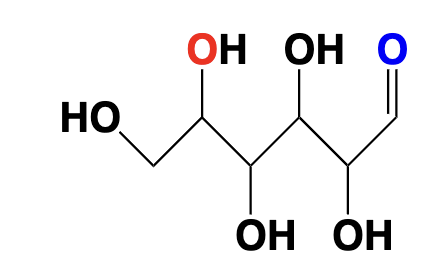
carbohydrate - multiple alcohols and a ketone/ aldehyde group
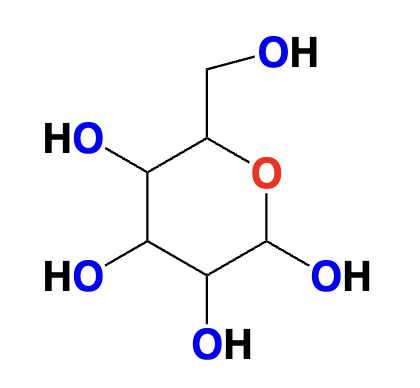
carbohydrate
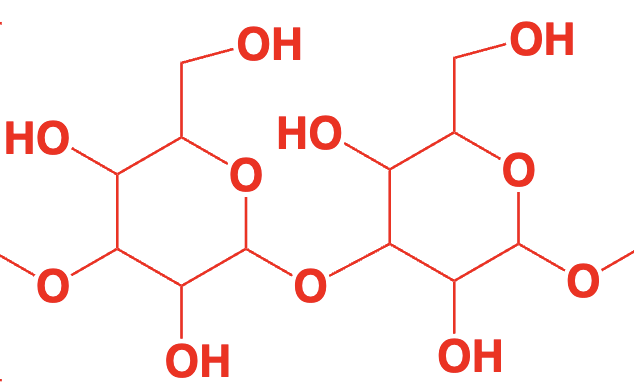
carbohydrate
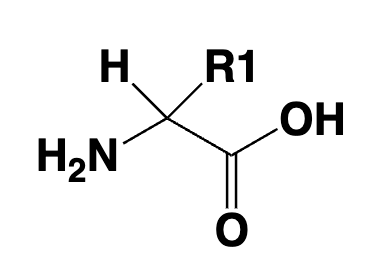
amino acid
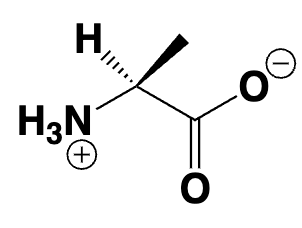
amino acid
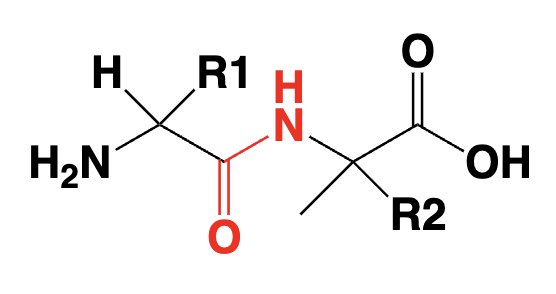
peptide/ protein
conjugated/ conjugated bonds
has alternating single/ double bonds OR has a resonance structure w/ alternating single/ double bonds (strong)
minor resonance contributor
higher in energy than the lowest energy resonance form (there may be multiple)
aromatic
cyclic
all atoms are planar (sp2 hybridized)
4n+2 electrons that belong to pi bonds
conjugated (including lone pairs/ vacant orbitals)
constitutional isomers
same molecular formula, but atoms are arranged differently (bonds are broken between the two isomers)
conformations/ conformers
different arrangements of atoms by rotating around a single bond;
there are anti, eclipsed, gauche, and eclipsed (least stable) conformers
steric strain
strain on a molecule that comes from atoms/ groups try and occupy the same space (meaning gauche is better than anti)
torsional strain
strain due to the twisting of a single bond
stereoisomers
molecules with the same bonds but different 3D geometry (like cis vs trans, axial vs equitorial)
enantiomers
mirror image molecules; aren’t able to be put on top of one another and overlap completely (opposite configs at all chiral centers)
must have chiral centers
diastereomers
have an opposite configuration at some chiral centers but not all
meso compounds
alpha D is zero
achiral compounds with chiral centers; they have a plane of symmetry
E substituents
higher priority is on opposite sides (~trans)
Z substituents
higher priority is on the same side (~cis)
IHD
[(2n+2)-A]/2
n = carbon
A = H + halogens - N - net charge
level of unsaturation
nucleophile
electron-rich species that bonds by donating a pair of electrons (lewis base)
electrophile
electron-poor species that accept a pair of electrons (vacant orbital, positive charge)
transition state
point where the bonds are in the process of breaking/ forming
-I (inductive) effect
a molecule with electronegative atom pulls the negative dipole towards it and decentralizes other electronegative charges (weakening base)
+I (inductive) effect
electron-releasing groups “push” negative charges towards electronegative atoms and increase polarity (strengthening base)
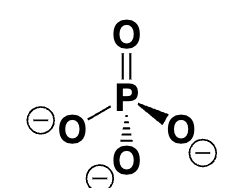
inorganic phosphate (pi)
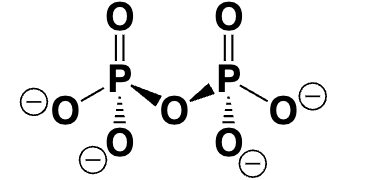
inorganic pyrophosphate (PPi)
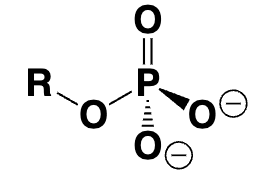
organic phosphate (R-OP)
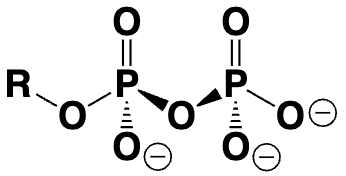
organic diphosphate (R-OPP)