8. metabolism
the energy of life
the cell is a miniature chemical factory where reactions occur
cellular respiration extracts energy stored in sugars and other fuels
cells apply this energy to perform work
some organisms convert energy to light - bioluminescence
metabolism transforms matter and energy
metabolism is the totality of an organism’s chemical reactions
it is an emergent property of life that arises from orderly interactions between molecules
not a physical reaction nor random
because viruses can’t metabolize, they are not alive
metabolic pathways
metabolic pathways - begins with a specific molecule and ends with a product
each step is catalyzed by a specific enzyme
each enzyme only works for that specific molecule and reactions - no enzyme can perform all the reactions in the metabolic pathway
each reaction is fueled by the reaction before it so it must go through all the reactions to reach the final product
catabolic pathways release energy by breaking down complex molecules
cellular respiration, the breakdown of glucose is catabolic
anabolic pathways consume energy to build complex molecules from simpler ones
bioenergetics is the study of how energy flows through living organisms
forms of energy
energy is the capacity to cause change
exists in various forms, some of which can perform work
kinetic energy - energy associated with motion
thermal energy - kinetic energy associated with random movement of atoms or molecules
heat - thermal energy in transfer between objects
potential energy - energy that matter possesses because of its location or structure
chemical energy - potential energy available for release in a chemical reaction
energy can be converted from one form to another
our bodies maintain heat because heat is a byproduct of energy conversion
laws of energy transformation
thermodynamics - the study of energy transformations
an isolated system is unable to exchange energy or matter with its surroundings (eg. thermos)
in an open system, energy and matter can be transferred
organism are open systems
laws of thermodynamics
energy can be transferred and transformed but it cannot be created no destroyed (principle of conservation of energy)
every energy transfer or transformation increases the entropy of the universe
entropy - measure of molecular disorder or randomness
during every energy transfer or transformation, some energy is unusable and is often lost as heat
living cells convert organized forms of energy to heat, a more disordered form of energy
spontaneous processes - occur without energy input
increases the entropy of the universe
releases energy -- catabolic process
nonspontaneous processes - will only occur if energy is provided
decreases entropy
absorbs energy to create order -- anabolic process
biological order and disorder
organisms create ordered structures from less organized forms of energy and matter
organisms also replace ordered from of matter and energy with less ordered forms
eg. animals consume complex molecules in food and release smaller, lower energy molecules and heat (catabolic)
cellular respiration is a catabolic reaction as it is the breaking down of glucose (order → disorder)
photosynthesis is an anabolic reaction since it converts energy into glucose (disorder → order)
the evolution of more complex organisms does not violate the second law of thermodynamics
entropy (disorder) may decrease in a particular system (photosynthesis) but the total entropy of the system and surroundings increases
free-energy change
in order to know which reactions occur spontaneously and which require the input of energy, they need to determine the energy and entropy changes that occur in chemical reactions
free-energy change ΔG
a living system’s free energy is energy that can do work when temperature and pressure are uniform as in a living cell (does not change even with a catalyst)
the change in ΔG during a process is related to the change in enthalpy
ΔG is negative for all spontaneous processes
spontaneous processes release energy
processes with 0 or positive ΔG are never spontaneous
spontaneous processes can be harnessed to perform work
free energy, stability and equilibrium
free energy - a measure of a system’s instability, its tendency to change to a more stable state
during a spontaneous change, free energy decreases and the stability of a system increases
equilibrium is a state of maximum stability
you die if you reach equilibrium
a process is spontaneous and can perform work only when it is moving towards equilibrium
more free energy (G) → less stable and greater work capacity
in a spontaneous change, the free energy of the system decreases (∆G < 0) → the system becomes more stable and the released free energy can be harnessed to do work
less free energy → more stable and less work capacity
free energy and metabolism
the concept of free energy can be applied to the chemistry of life’s processes
exergonic reactions - proceed with a net release of free energy and is spontaneous
negative ∆G
catabolic reaction
as the reaction proceeds, energy is released meaning the free energy of the products is LESS than that of the reactants (∆G = products - reactants)
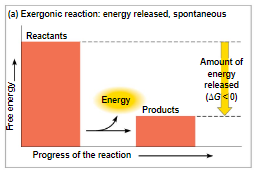
endergonic reactions - absorb free energy from its surroundings and is nonspontaneous
positive ∆G
anabolic reaction
as the reaction proceeds, energy is absorbed from the surroundings meaning the free energy of the products is GREATER than that of the reactants
equilibrium and metabolism
reactions in a closed system eventually reach equilibrium and can then do no work
cells are not in equilibrium -- they are open systems experiencing a constant flow of materials
a defining feature of life is that metabolism is never at equilibrium
a catabolic pathway in a cell releases free energy in a series of reactions
ATP powers cellular work
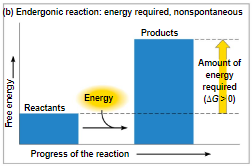
a cell does three main kinds of work
chemical work - pushing endergonic reactions
transport work - pumping substances against the direction of spontaneous movement
mechanical work - contraction of muscle cells
to do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one
most energy coupling in cells is mediated by ATP
the structure and hydrolysis of ATP
ATP (adenosine triphosphate) is the cell’s energy shuttle
it is composed of ribose (sugar), adenine (nitrogenous base), and 3 phosphate groups
cellular respiration exists to form ATP
catabolic and exergonic since it breaks down glucose
the bonds between the phosphate groups of ATP’s tail can be broken down by hydrolysis
energy is released when the terminal phosphate bond is broken
this release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves
how the hydrolysis of ATP performs work
the 3 types of cellular work are powered by the hydrolysis of ATP
in the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction
the coupled reactions are exergonic overall since there is left over energy bc they are not perfectly efficient
ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to some other molecule
the recipient molecule is now a phosphorylated intermediate
phosphorylated - means phosphorus is added to it
transport and mechanical work in the cell are also powered by ATP hydrolysis
ATP hydrolysis leads to a change in protein shape and binding ability
active transport - requires energy
can go against the concentration gradient
transports proteins that are too large
passive transport - does not require energy
the regeneration of ATP
ATP is a renewable resource that is regenerated by the addition of a phosphate group to adenosine diphosphate (ADP)
the energy to phosphorylated ADP comes from catabolic reactions in the cell
ADP + phosphorus = anabolic
the ATP cycle is a revolving door through which energy passes during its transfer from catabolic and anabolic pathways
using ATP in cellular respiration is catabolic because it breaks down into ADP and the phosphorylated intermediate
enzymes speed up metabolic reactions
a catalyst is a chemical agent that speeds up a reaction without being consumed by it
an enzyme is a catalytic protein
eg. sucrase is an enzyme that catalyzes the hydrolysis of sucrose
needs energy 99% of the time to work
every chemical reaction between molecules involves bond breaking and bond forming
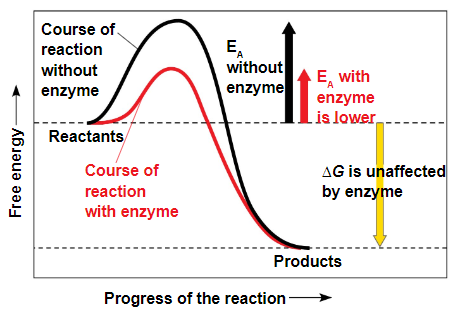
the initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (Eₐ)
activation energy is often supplied in the form of thermal energy that the reactant molecules absorb from their surroundings
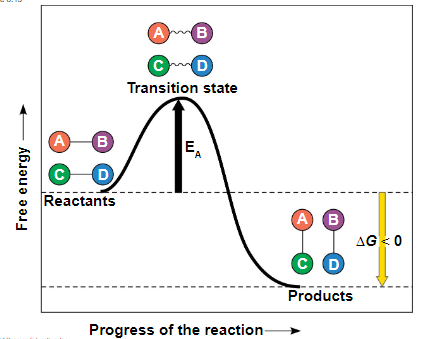
because ∆G is negative, it is an exergonic reaction
the bump in the graph is the activation energy
catalysts reduce the EA
enzymes do not change ∆G because if it does, it is taking part in the reaction
in catalysis, enzymes or other catalysts speed up specific reactions by lowering the EA barrier
substrate specificity of enzymes
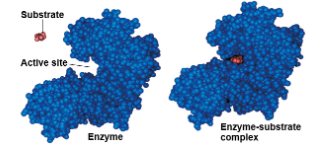
the reactant that an enzyme acts on is called the enzyme’s substrate
enzyme puts pressure on the bonds within the substrate to break it down into different sections
energy released from breakdown is ∆G
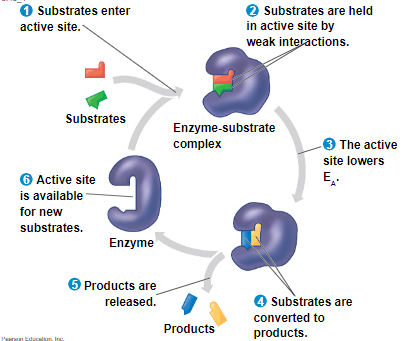
the enzyme binds to its substrate, forming an enzyme-substrate complex
while bound, the activity of the enzyme converts substrate to product
the reaction catalyzed by each enzyme is very specific
the active site is the region on the enzyme where the substrate binds
induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction
in an enzymatic reaction, the substrate binds to the active site of the enzyme
enzymes are extremely fast acting and emerge from the reactions in their original form
in the long term, structure doesn't change
very small amounts of enzymes can have huge metabolic effects because they are used repeatedly in catalytic cycles
in prokaryotes, if half of the substrate is rare and the other is abundant, the full substrate cannot be made
the active site can lower an EA barrier by:
orienting substrates correctly
straining substrate bonds
providing favorable microenvironments
covalently bonding to the substrate
the rate of an enzyme-catalyzed reaction can be sped up by increasing substrate concentrations
when all enzyme molecules have their active sites engages, the enzyme is saturated
if the enzyme is saturated, the reaction rate can only be sped up by adding more enzymes
effects of local conditions on enzymes
an enzyme’s activity can be affected by
general environmental factors such as temp and PH
chemicals that specifically influence the enzyme
levels of enzyme activity will change
each enzyme has an optimal temperature in which it can function or else it will be denatured
each enzyme has an optimal pH in which it can function
this is why our bodies have buffers
optimal conditions favor the most active shape for the enzyme molecule
cofactors
cofactors - non-protein enzyme helpers
may be inorganic (like a metal) or organic
an organic cofactor is called a coenzyme
eg. vitamins
they attach in the passive site (allosteric site) to help shape enzymes for certain substrates
active site - where the substrate goes
allosteric site - where the coenzyme goes
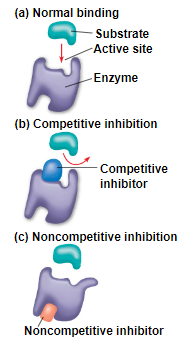
inhibitors
competitive inhibitors - bind to the active site of an enzyme, competing with the substrate
has the same shape as the substrate to inhibit the proper substrate from binding to the enzyme
non-competitive inhibitors - bind to the passive site of the enzyme causing the enzyme to change shape and make the active site less effective
eg. toxins, poison, pesticides, antibodies
like how tiktok inhibits people from doing work
opposite of inhibitor is activator
when the effects on an inhibitor can be reversed by adding more substrate, it is a reversible inhibitor
the evolution of enzymes
enzymes are proteins encoded by genes
changes in genes lead to changes in amino acid composition of an enzyme
altered amino acids, particularly at the active site, can result in novel enzyme activity or altered substrate specificity
under environmental conditions where the new function is beneficial, natural selection would favor the mutated allele
regulation of enzyme activity
chemical chaos would result if a cell’s metabolic pathways were not tightly regulated
a cell does this by switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes
allosteric regulations
allosteric regulation may either inhibit or stimulate an enzyme’s activity
allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
most allosterically regulated enzymes are made from polypeptide subunits, each with its own active site
the enzyme complex has active and inactive forms
the binding of an activator stabilizes the active form of the enzyme
the binding of an inhibitor stabilizes the inactive form of the enzyme
eg. some drugs inhibit enzymes from doing its job
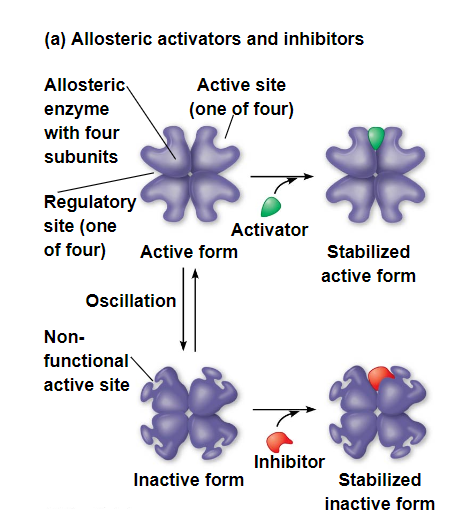
cooperativity - a form of allosteric regulation that can amplify enzyme activity
one substrate molecule primes an enzyme to act on additional substrate molecules more readily
cooperativity is allosteric because binding by a substrate to one active site affects catalysis in a different active site
in feedback inhibition, the end product of a metabolic pathway shuts down the pathway
prevents a cell from wasting chemical resources by synthesizing more product than needed
eg. a thermostat tells the furnace when to stop once the temperature is high or low enough
negative inhibition is good for the body
positive inhibition ONLY occurs during pregnancy
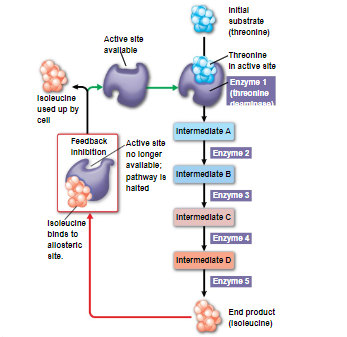
only happens when the product is in excess otherwise, it will get used up
localization of enzymes within the cell
structures within the cell help bring order to metabolic pathways
some enzymes act as structural components of membranes
in eukaryotic cells, some enzymes reside in specific organelles
eg. enzymes for cellular respiration are located in mitochondria