Unit #3 Test - Mrs. Williams
1/26
Earn XP
Description and Tags
GSCI-2010-01: Physical Science for Educators
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
What is an Atom?
The basic building block of matter
What is an Element?
Matter made up of only one kind of atom
What is a Molecule?
Two or more atoms bonded together
Parts of an Atom
Protons (+), neutrons, electrons (-)
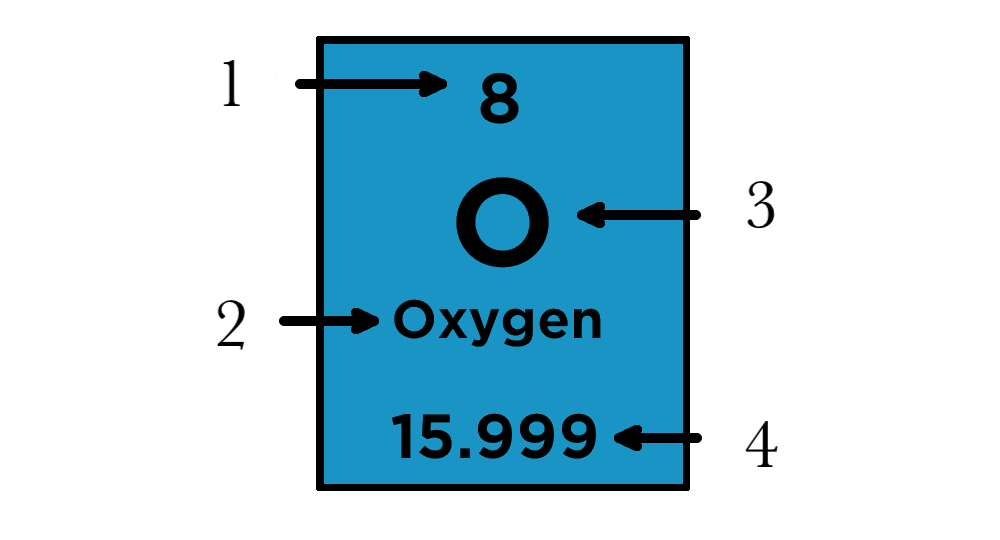
How to Read a Box of the Periodic Table
Atomic number
Element Name
Elemental Symbol
Atomic Weight
Calculate the Number of Protons, Neutrons, and Electrons
Protons - Atomic Number
Neutrons - Atomic Number - Atomic Mass
Electrons - Atomic Number
What is a Compound?
A pure substance whose smallest unit is made up of two different atoms chemically bonded together
What is a Mixture?
A combination of two or more substances
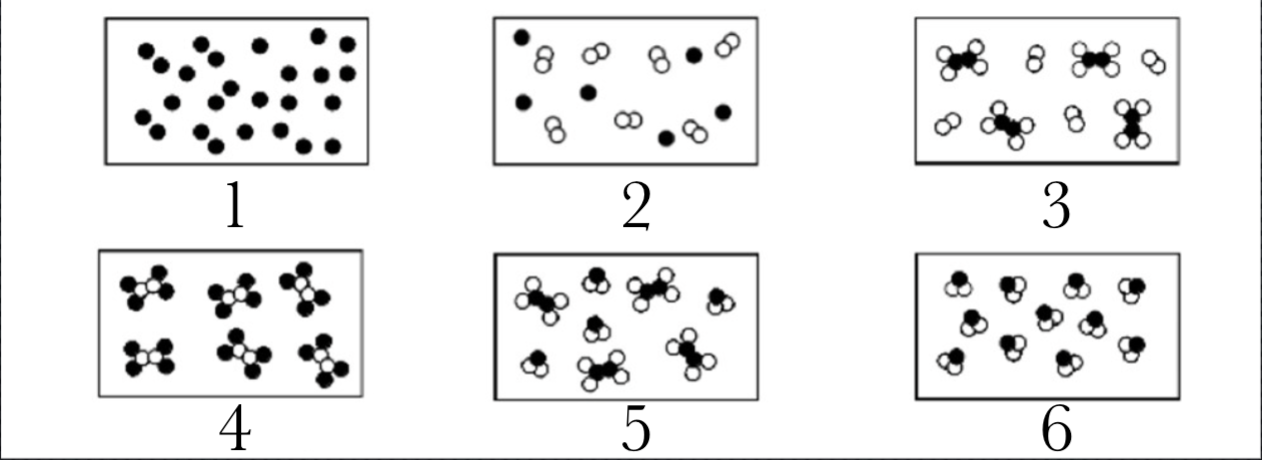
Identify Molecules, Compounds, and Mixtures
Molecule
Mixture
Mixture
Compound
Mixture
Compound
Periods vs. Groups
Periods
Horizontal
Share similar physical/chemical properties
Groups
Vertical
Each new row indicates a new electron shell
Physical vs. Chemical Changes
Physical - When matter changes visual form
Chemical - Result in the production of a new substance and cannot be reversed
Conservation of Mass
Mass cannot be created or destroyed in a chemical reaction
What is Matter?
Anything that takes up space and has mass
States of Matter
Solid, liquid, and gas
Atomic Level of Solids
Contain tightly packed atoms and a fixed shape
Atomic Level of Liquids
Contain loosely packed atoms and no fixed shape
Atomic Level of Gases
Contain rapidly moving atoms and far apart
How Solids Change State
Melting and sublimation
How Liquids Change State
Freezing and evaporation
How Gases Change State
Deposition and condensation
How to find Volume in a Graduated Cylinder
Subtract the submerged object water line from the initial water line
How to find Volume in a Displacement Vessel
The amount of spilled water
What is Density?
The amount of mass per unit of volume
Equation for Density
Density = Mass/Volume
Solvent vs. Solute
Solvent - Part of the solution that is present in the largest amount
Solute - The minor part of a solution and is dissolved by the solvent
Acids vs. Bases
Acids
Sour taste
Corrosive to metals
Bases
Bitter taste
Slippery when wet
Often used for cleaning
pH Scale
Measurement of the concentration of hydrogen ions and range from 0-14 with 0-7 being acids and 7-14 are bases