biochem ch4 amino acids and peptide bonds
1/107
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
108 Terms
amino acids have what kinds of carbon
central tetrahedral carbon
what are the bonds between amino acids
peptide bonds
how many common AA are there
20
are all amino acids found in proteins
no
what structure do most amino acids pocess , except for proline
most amino acids are (referring to central carbon)
chiral
what does it mean for a carbon to be chiral
has 4 different substituents and it is tetrahedral
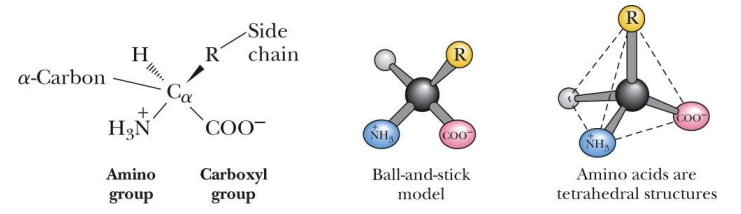
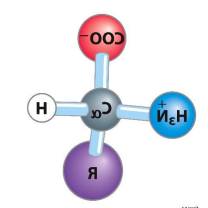
all amino acids have what 3 groups and have what shape
an acidic carboxyl group
a basic amino group
an a-hydrogen connected to the a-carbon
tetrahedral shape
which amino acid is exception for last flashcard
proline
what is the R group
unique 4th substituent of an amino acid that
glycine (chirality, R group)
not chiral as R group is H
-so has central carbon has 2 H groups
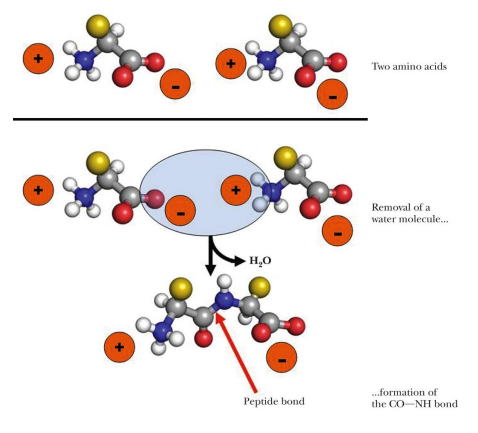
how do amino acids form peptide bonds
by dehydration to form covalent bond
what are the 5 classes of an amino acid
nonpolar, aliphatic
aromatic
polar neutral
acidic
basic
what are nonpolar AA’s (characteristics)
nonpolar so love fat
they are aliphatic so sidechain has open structure
what are the nonpolar AA’s
Glycine
alanine
valine
leucine
isoleucine
methionine
proline
glycine (1 letter, 3 letter)
G, Gly
alanine (1 letter, 3 letter)
A, Ala
valine (1 letter, 3 letter)
V, Val
proline (1 letter, 3 letter)
P, Pro
Leucine (1 letter, 3 letter)
L, Leu
Isoleucine (1 letter, 3 letter)
I, Ile
Methionine (1 letter, 3 letter)
M, Met
aromatic AA’s polarity
mostly nonpolar, except for tyrosine which has slight polarity due to OH group
aromatic AA UV
270-280nm, Allows us to quantify protein concentrations (Spectrophotometry)
what are the aromatic AAs
Phenylalanine
Tyrosine
Tryptophan
Phenylalanine (1 letter, 3 letter)
F, Phe
tyrosine (1 letter, 3 letter)
Y, Tyr
Tryptophan (1 letter, 3 letter)
W, Trp
polar uncharged AAs can form what bond? what about cys?
hydrogen bonds and cysteine forms disulfide bonds
what are the polar uncharged AAs
Serine
Cysteine
Threonine
glutamine
asparagine
Serine (1 letter, 3 letter)
S, Ser
Cysteine (1 letter, 3 letter)
C, Cys
Threonine (1 letter, 3 letter)
T, Thr
Glutamine (1 letter, 3 letter)
Q, Gln
Asparganine (1 letter, 3 letter)
N, Asn
acidic amino acids have what charge
polar with negative charge (carboxyl is negative)
what are the acidic amino acids
glutamate
aspartate
aspartate (1 letter, 3 letter)
D, Asp
Glutamate (1 letter, 3 letter)
E, Glu
basic amino acids have what charge
polar with positive charge (amine is positive)
what are the polar basic amino acids
lysine, arginine, histidine
lysine (1 letter, 3 letter)
K ,Lys
Arganine (1 letter, 3 letter)
R, Arg
Histidine (1 letter, 3 letter)
H, His
what are uncommon amino acids
Not incorporated by ribosomes
how do uncommon amino acids come to be (how are they made (4), what is the most common)
Arise by post-translational modifications of proteins
phosphorylation
hydroxylation
methylation
acetylation
Reversible modifications, especially phosphorylation (kinases/phosphatases), are important in regulation and signaling (MOST COMMON)
selenocysteine is found in, made by, why is it important?
Selenocysteine has been found in many organisms
Half of eukaryotes and most bacteria contain selenoproteins
Selenocysteine is the only common amino acid that humans can make but higher plants cannot
Replacement of sulfur with selenium creates molecules more resistant to oxidation
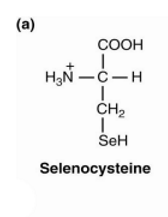
human selenoenzymes are involved in
peroxide removal,
reduction of thioredoxins (The thioredoxin ((Trx) system is one of the central antioxidant systems in mammal cells)
selenophosphate synthesis,
activation and inactivation of thyroid hormones,
Repair of oxidized Met in protein
pyrrolysine found in
several archeal species
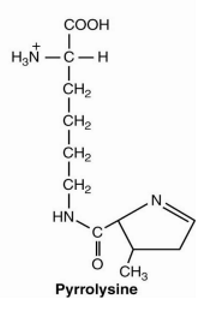
Hydroxylysine, hydroxyproline are found in
collagen
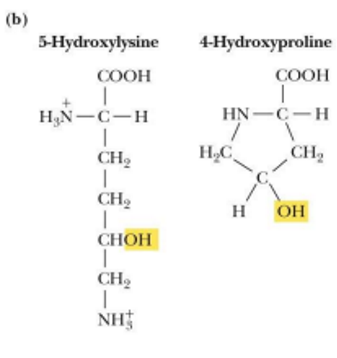
carboxygltamate is a ______________ protein
blood clotting
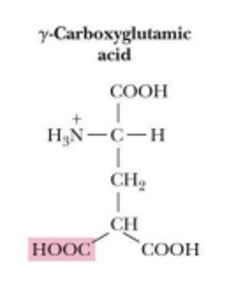
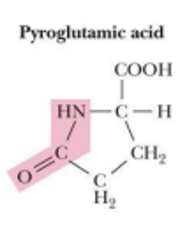
pyroglutamate is found in
in bacteriorhodopsin (is a archeal protein in halobacteria that acts as a protein pump)
phosphorylated amino acids act as a
signaling device
what role do amino acid derivatives play (2)
act as neurotransmitters and hormones
acidic PH means
Basic pH means
Neutral pH means
acidic = (↑H+)
basic = (↑OH-)
neutral = pH 7
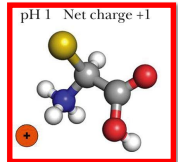
at acidic pH, what happens to amino acids
the carboxyl group becomes pronated since it was either neutral or negative before
this turns the amino acid into a cation (positive charged) since the amine group is already positive or neutral
at neutral pH, what happens to amino acid
the carboxyl group becomes deprotonated (negative) and the amine group becomes pronated (+) so the net charge is 0 (neutral)
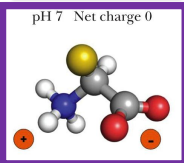
when a amino acid has a net charge of 0, it is called
zwitterions
-also can be called amphoteric or ampholyte
what are zwitterions (&function)
when in pI
can act as a acid OR a base
in a alkaline pH, what happens to amino acids
the amine group becomes neutral (NH2) (as it was positive before and lost a H+)
-the amino acid is now in anionic form (because the carboxyl group is negative, so that makes an overall negative charge)
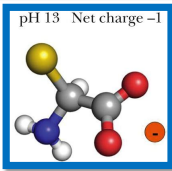
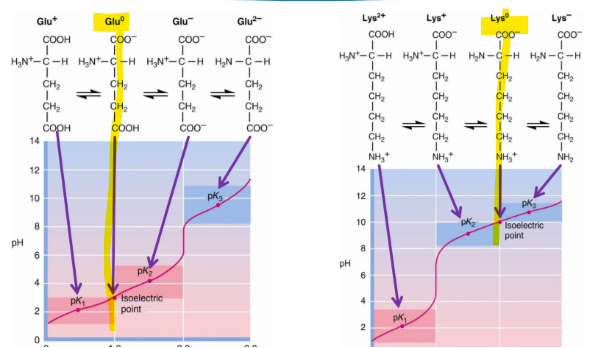
what is the isoelectric point
is the pH at which the average charge on the molecule is zero
when at zwitterion
for a simple amino molecule, the pI is found how
average of the two pKa values surrounding the isoelectric species
Since amino acids has 2 or more groups that can be pronated (the carboxyl and amine group), it has 2 pka (or more)
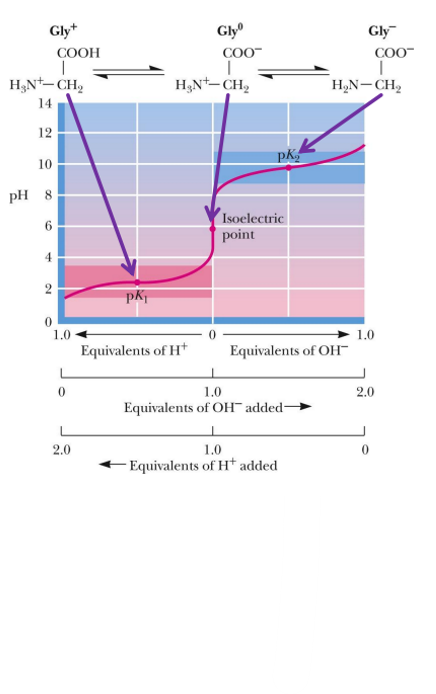
pK is what
the tendency to give up a proton
isoelectric point of glycine
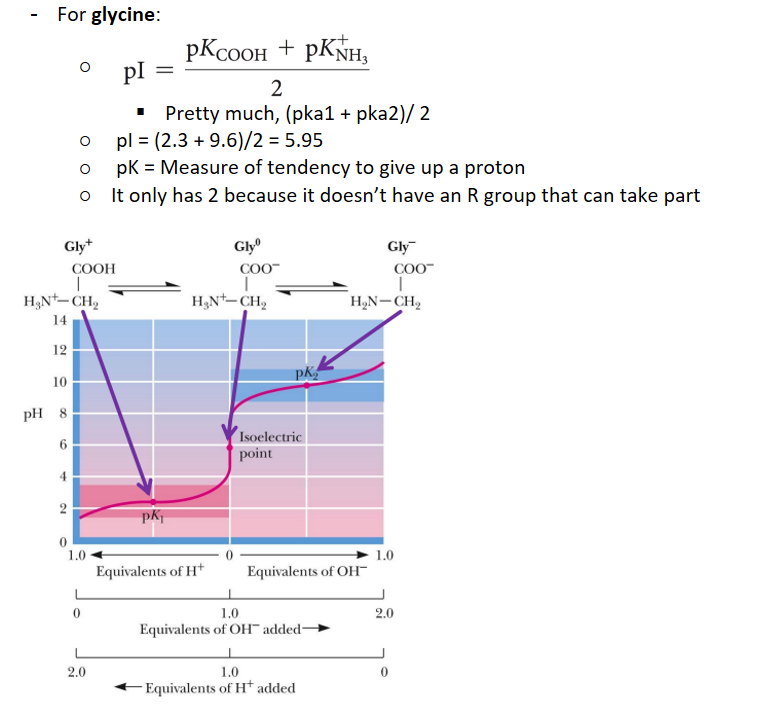
what is a tritation curve
a graphical representation of the pH of a solution in a tritration
To the left of the pI, we have added H+, to the right of the pI we have taken away H+. So this means that the more left the more positive, the more right, the more negative
when having to figure out the titration curve, look for when the amino acid is at 0, and that is the pI
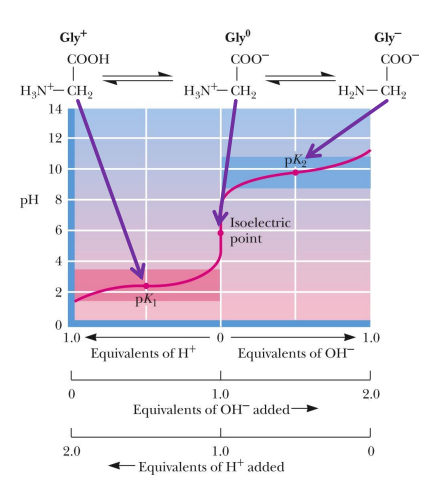
ionizable side chains can also be
tritiated (because they can be pronated or deprotonated)
when an amino acid has a ionizable side chain, how does this affect the titration curve
Titration curves are now more complex
Three pKa values
Because of R groups that can also be pronated or depronated
pKa values are discernable if two pKa values are more than two pH units apart
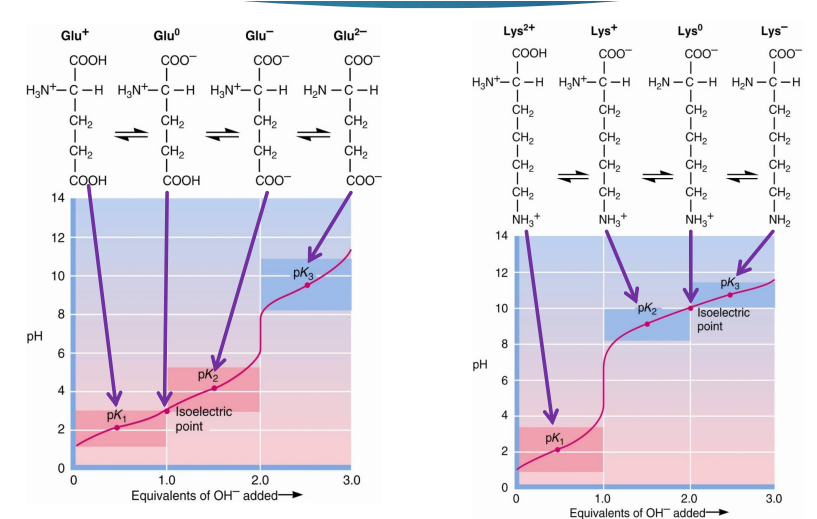
if given a titration curve of a ionizable amino acid, how do you find the pI
find the pk1 and pk2 and add those, then divide by 2
OR
just find when the amino acid charge is 0
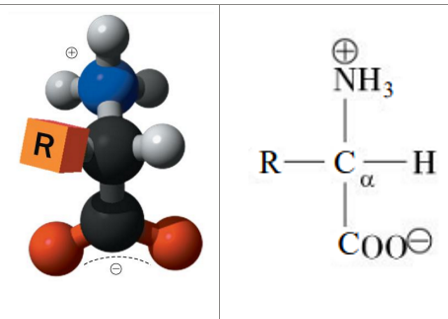
what makes something chiral and aka’s (2)
has 4 different groups attached to it
aka - stereocenter, or asymmetrical carbon
Fischer projection vs perspective projection
Fischer Projections:
Horizontal bonds: out of plane;
Vertical bonds: behind plane
Perspective formulas:
Dashed bonds – behind plane
Solid bonds – out of the plane
what are the 4 types of isomers
stereoisomer
geometric isomers
enantiomers
diastereomers
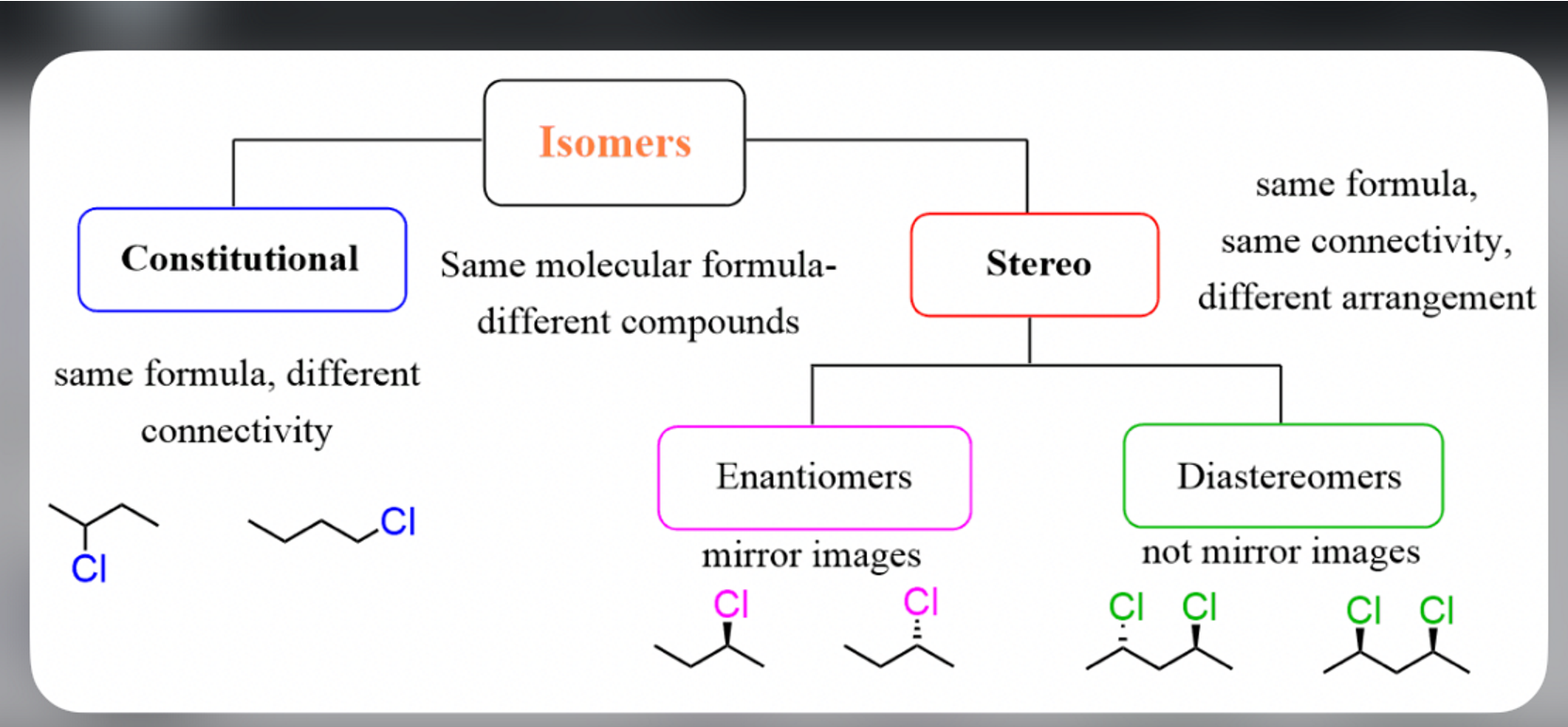
stereoisomers have (same/dif in what properties )
different physical properties
these then break down into more specific types of stereoisomers
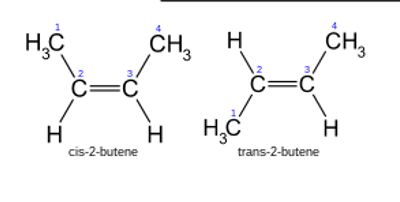
geometric isomers are also called? differ or same in what properties ?
aka cis and trans
different physical and chemical properties
enantiomers are what? same or different in what properties ?
mirror images
SAME chemical properties BUT differ in CERTAIN physical and biological properties
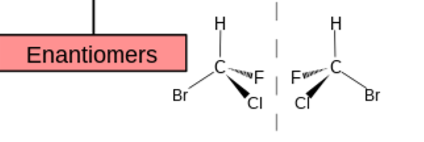
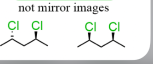
diastereomers are what and differ or same in what properties
not mirror images
DIFF physical and chemical and biological properties
all amino acids are chiral except for
glycine (has H as R group)
what are the 2 strutures of amino acids (think letters)
L and D
D,L nomenclature (what is what, what groups go where)
based on glyceraldehyde
COO- group is on top
R group on bottom
Is D is amine group is on right side
is L if amine group is on left side
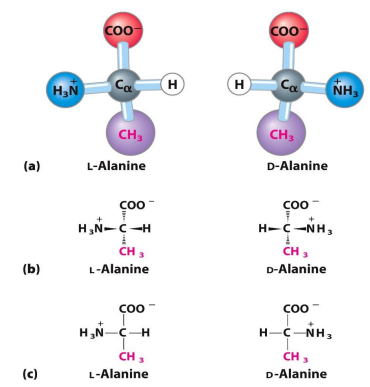
which type of D,L nomenclature in amino acids is mainly seen in nature
L-amino acids
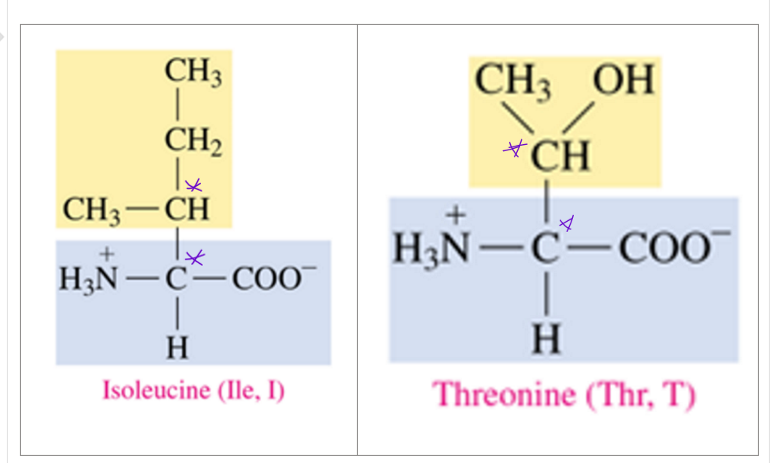
what 2 amino acids have 2 chiral centers
Ile and Thr
how do we name using R,S system (what is the main focus)
by viewing the molecule from the chiral center to the atom with the lowest priority.
r,s system is used to identify the absolute configuration of enantiomers (r stands for right and S for left- way the arrows go)
The priorities of the functional groups are:
SH > OH > NH2 > COOH > CHO > CH2OH > CH3
Thiol> alcohol> amine> carboxyl> aldehyde> hydroxymethyl> methyl> hydrogen
So we first assign priority to all groups except the chiral carbon. We use the top 3 priorities. We write the one with highest priority, then you place the other group in order based on priority, but if it is L, you do it counterclockwise and it is an S system. If it is D, then you write them clockwise and it is a R system. You write arrow starting at highest priority in the order it goes.
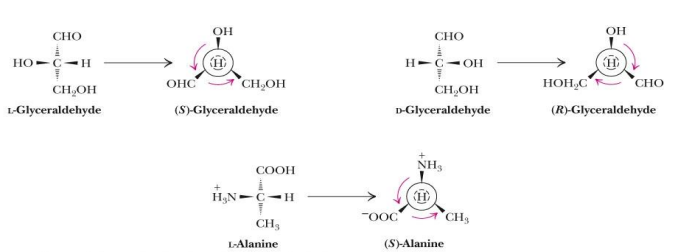
what are the priorities of the functional groups
SH > OH > NH2 > COOH > CHO > CH2OH > CH3
Thiol> alcohol> amine> carboxyl> aldehyde> hydroxymethyl> methyl> hydrogen
all amino acids absorb what kinds of light
infrared
what amino acid absorb UV ligth
Only Phe, Tyr, and Trp absorb UV
Absorbance at 280 nm is a good diagnostic test for amino acids
Rmb how nucleotides absorb at 250-270nm)
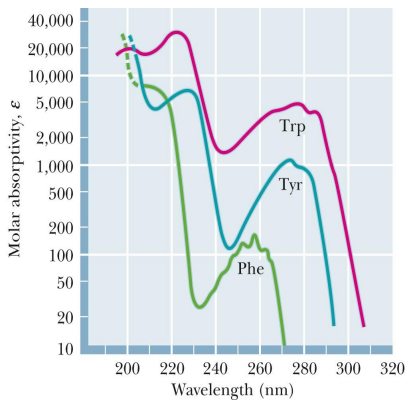
what does chromatography do (really basic function)
separation of amino acid mixtures
proteins are ___________ polymers
unbranched
how do amino acids bond together
N terminal to C terminal using peptide bonds h
how do we read an amino acid sequence
N terminus to C terminus
how is the peptide bond formed
dehydration
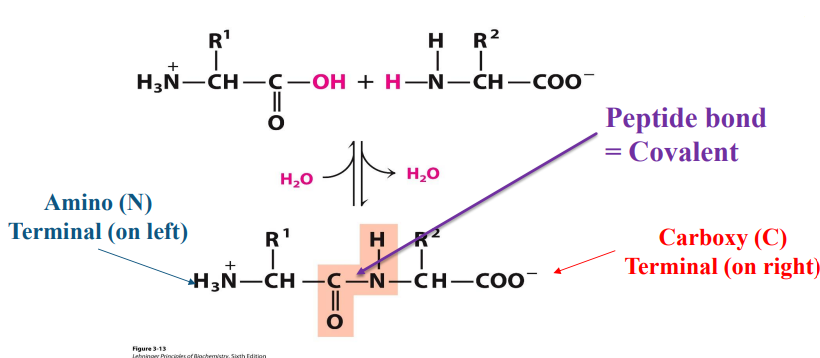
the peptide backbone of a protein consist of repeated sequences of : (hint, not amino acids but like parts of them)
–N-Cα -Co -
“N” is the amide nitrogen of the amino acid
“Cα ” is the alpha-C of the amino acid
“Co ” is the carbonyl carbon of the amino acid
when amino acids link up to a chain, they are called a
peptide
each amino acid unit is called a
residue
dipeptide
tripeptide
oligopeptide
polypeptide
(number wise; each one )
dipeptide- 2 residues
tripeptide- 3 residues
oligopeptide- 12 to 20 residues
polypeptide- >15-20 residues
what is a polypeptide (MW)
10,000
a protein (MW to make it one)
> 10,000
peptide are usually in what conformation
trans conformation
peptide bond has a large (type of force)
dipole moment
why does peptide have a large dipole moment
N partially positive; O partially negative
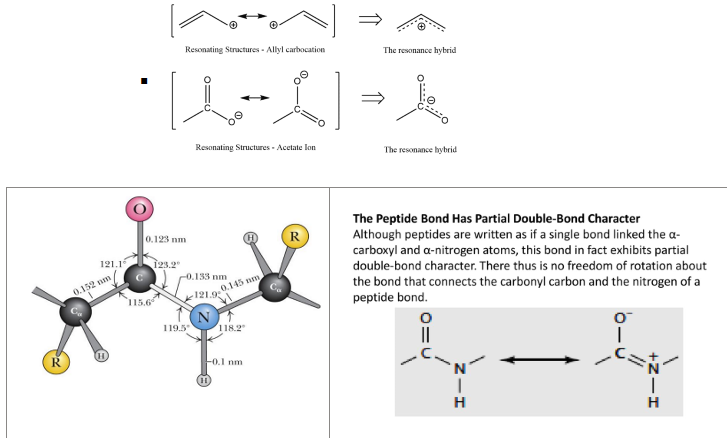
length of peptide bond
Has a length of about 0.133 nm - shorter than a typical single bond but longer than a double bond
what do we mean by peptide bond having a resonance hybrid
Resonance hybrid- the electron within a structure change constantly due to movement of bonds, so molecule represented by more than one Lewis structures, these combined give a resonance hybrid
SOOOOOOO
this means that in the peptide bond, the carboxyl and Nitrogen atom (within the peptide bond) kinda go back and forth where the C=O bond then become C=N bond and vice versa. This gives the peptide bond a partial double bond character and an amide plane (limiting rotation between the C (of carbonyl) and N bond