BioChem Exam 2 PP
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Account for the origin of the term carbohydrate
Carbohydrates were originally regarded as hydrates of carbon because the empirical formula of many of them is (CH2O)n
-name comes from carbon and hydrate (water) since for every C atom there is one water molecule in the formula
Indicate whether each of the following pairs of sugars consists of anomers, epimers, or an aldose–ketose pair.
a. D-glyceraldehyde and dihydroxyacetone
b. D-glucose and D-mannose
c. D-glucose and D-fructose
d. 𝛼-D-glucose and 𝛽-D-glucose
e. D-ribose and D-ribulose
f. D-galactose and D-glucose
a. aldose–ketose;
b. epimers;
c. aldose–ketose;
d. anomers;
e. aldose–ketose;
f. epimers
To which classes of sugars do the monosaccharides shown here belong? (USE PHOTO)
Erythrose: tetrose aldose.
Ribose: pentose aldose.
Glyceraldehyde: triose aldose.
Dihydroxyacetone: triose ketose.
Erythrulose: tetrose ketose.
Ribulose: pentose ketose.
Fructose: hexose ketose.

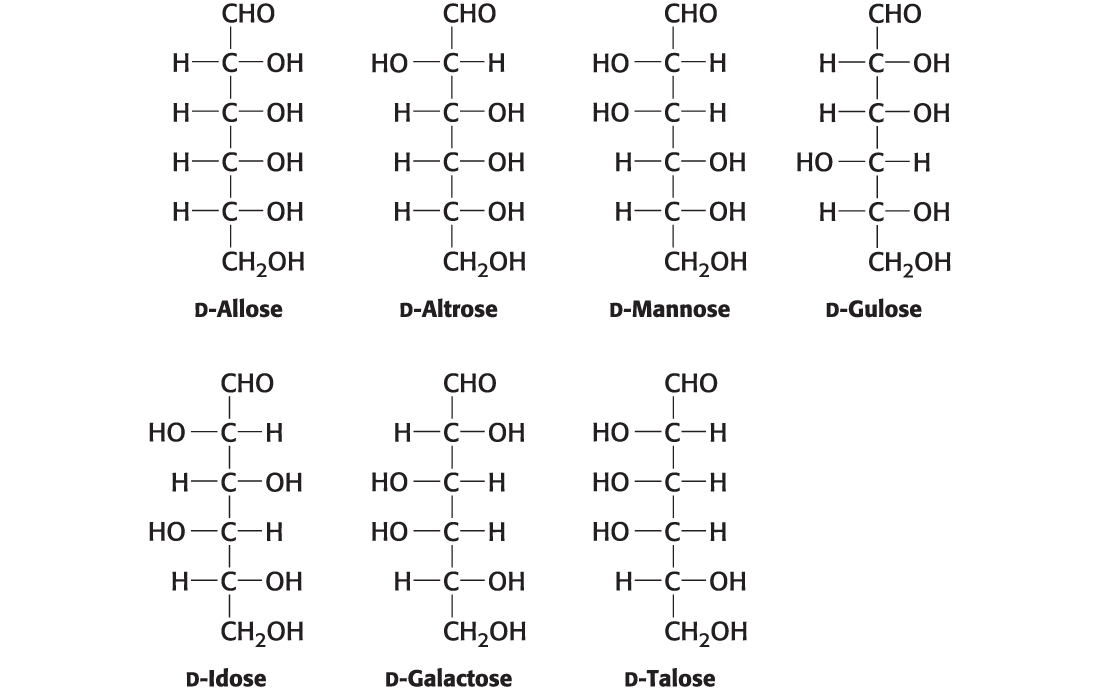
Although an aldose with four asymmetric carbon atoms is capable of forming 16 diastereoisomers, only eight of the isomers are commonly observed, including glucose. They are listed after the structure of D-glucose with their structural relation to glucose. Using the structure of glucose as a reference, draw the structure.
What are lipids?
Lipids are water-insoluble molecules that are highly soluble in organic solvents.
Triacylglycerols are used for fuel storage in both plants and animals. The triacylglycerols from plants are often liquid at room temperature, whereas those from animals are solid. Suggest some chemical reasons for this difference.
Triacylglycerols from plants may have many cis double bonds or have shorter fatty acid chains than those from animals.
Distinguish between phosphoglycerides and triacylglycerols.
Triacylglycerols consist of three fatty acid chains attached to a glycerol backbone. Triacylglycerols are a storage form of fuel. Phosphoglycerides consist of two fatty acid chains attached to a glycerol backbone. The remaining alcohol of the glycerol is bonded to a phosphate, which in turn is bonded to an alcohol. Phosphoglycerides are membrane components.
Arrange the following substances in order of increasing permeability through a lipid bilayer: (a) glucose; (b) glycerol; (c) Cl-; (d) indole; (e) tryptophan.
(c), (a), (e), (b), (d)
Differentiate between simple diffusion and facilitated diffusion.
In simple diffusion, the molecule in question can diffuse down its concentration gradient through the membrane. In facilitated diffusion, the molecule is not lipophilic and cannot directly diffuse through the membrane. A channel or carrier is required to facilitate movement down the gradient.
Differentiate between passive transport and active transport.
In passive transport (facilitated diffusion), a substance moves down its concentration gradient through a channel or transporter. In active transport, a concentration gradient is generated at the expense of another source of energy, such as the hydrolysis of ATP.
What are two fundamental properties of all ion channels?
Selectivity and the rapid transport of ions.
Differentiate between ligand-gated and voltage-gated channels.
Ligand-gated channels open in response to the binding of a molecule by the channel, whereas voltage-gated channels open in response to changes in the membrane potential.
List two forms of energy that can power active transport.
The two forms are (1) ATP hydrolysis and (2) the movement of one molecule down its concentration gradient coupled with the movement of another molecule up its concentration gradient.
What are the three major classes of membrane receptors?
(1) G-protein coupled (seven-transmembrane-helix) receptors; (2) receptors that dimerize on ligand binding and recruit tyrosine kinases; (3) receptors that dimerize on ligand binding that are tyrosine kinases (receptor tyrosine kinases).
Explain how a small number of hormones binding to the extracellular surface of a cell can have a large biochemical effect inside the cell.
The initial signal—the binding of the hormone by a receptor—is amplified by enzymes and channels.
What are some of the structural features common to all membrane-bound receptors?
The receptor must have a site on the extracellular side of the membrane to which the signal molecule can bind and must have an intracellular domain. Binding of the signal to the receptor must induce structural changes on the intracellular domain so that the signal can be transmitted.
Why is the GTPase activity of G proteins crucial to the proper functioning of a cell?
The GTPase activity terminates the signal. Without such activity, after a pathway has been activated, it remains activated and is unresponsive to changes in the initial signal.
How would a lack of bile salts affect digestion?
Lipid digestion and absorption would be hindered, and much lipid would be excreted in the feces.
Differentiate between anabolism and catabolism.
Anabolism is the set of biochemical reactions that use energy to build new molecules and, ultimately, new cells. Catabolism is the set of biochemical reactions that extract energy from fuel sources or break down biomolecules.
What factors account for the high phosphoryl-transfer potential of nucleoside triphosphates? 6
Charge repulsion, resonance stabilization, increase in entropy, and stabilization by hydration.
Why does it make good sense to have a single nucleotide,
ATP, function as the cellular energy currency?
Having only one nucleotide function as the energy currency of the cell enables the cell to monitor its energy status.
The conversion of one molecule of fructose 1,6-bisphosphate into two molecules of pyruvate results in the net synthesis of:
two molecules of NADH and four molecules of ATP
The conversion of one molecule of glucose into two molecules of lactate results in the net synthesis of:
No NADH and two molecules of ATP.
Which of the following statements is (are) true for a muscle performing lactic acid fermentation? 3
The process is inhibited by ATP.
The process is exergonic.
Each of the following molecules is processed by glycolysis to lactate. How much ATP is generated from each molecule?
a. Glucose 6-phosphate
b. Dihydroxyacetone phosphate
c. Glyceraldehyde 3-phosphate
d. Fructose
e. Sucrose
a. 3 ATP;
b. 2 ATP;
c. 2 ATP;
d. 2 ATP;
e. 4 ATP
What energetic barrier prevents glycolysis from simply running in reverse to synthesize glucose? What is the energetic cost of overcoming this barrier?
The reverse of glycolysis is highly endergonic under cellular conditions. The expenditure of six NTP molecules in gluconeogenesis renders gluconeogenesis exergonic.
Liver is primarily a gluconeogenic tissue, whereas muscle is primarily glycolytic. Why does this division of labor make good physiological sense?
Muscle is likely to produce lactic acid during contraction. Lactic acid is a strong acid and must not accumulate in muscle or blood. Liver removes the lactic acid from the blood and converts it into glucose. The glucose can be released into the blood or stored as glycogen for later use.