Chemistry Aromatic Compounds
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
importance fo aromatic compounds
solvents are building blocks for polymers, they are components of some amino acids and contribute to the structure of DNA.
name of aromatic compounds
arenes, parent compound is benzene ring from the phenyl functional group
aromatic hydrocabron groups named aryl
naming aromatic compounds
for bond to first benzene ring, suffix ortho
for bond to second benzene ring, suffix meta
for bond to third benzene ring, suffix para
for compounds around which carbon in benzene ring:
numbering the carbon atoms from 1 to 6 around the ring
arenes and electrophilic substitution
they are unsaturated, but not reactive despite double bonds. They underdo electrophilic substitution reactiont o keep pi ring in tact
typically under teh use of teh catalyst; iron(III) bromide, which acts as a Lewis acid
electrophilic substitution
electrophilic attack on an atom and the replacement of one atom by another or by a group of atoms.
begins the same as electrophilic addition, an attack on a region of high electron density to form a positively charged intermediate. hwoever due to electron ring stability, hydrogen atom is pulled from the ring as H+ and stability is returned through substitution. THis is where catalyst is also released, able to react again
stability from delocalised pi electrons
Delocalization gives the electrons such low energy, they are bound so tightly, that they are unavailable for forming new sigma-bonds
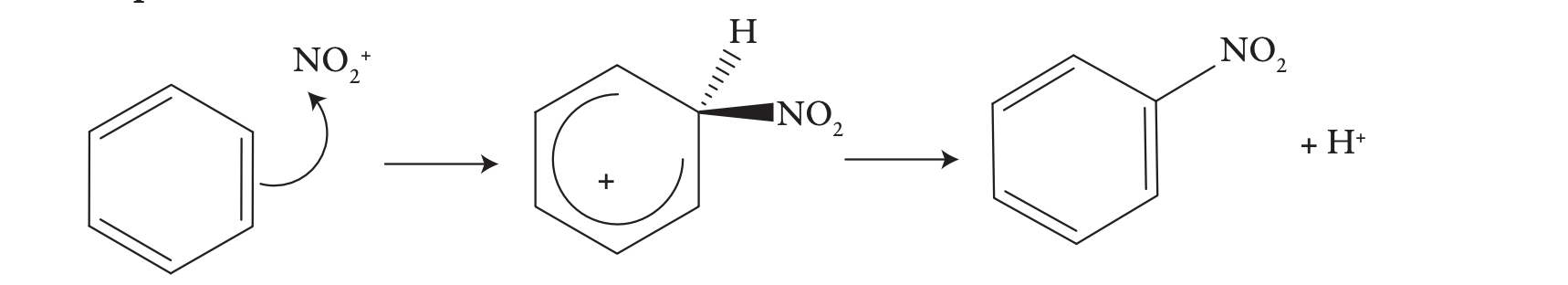
nitration of benzene
A mixture of nitric acid and concentrated sulfuric acid converts benzene slowly into nitrobenzene.
electrophile NO2+ the nitrating agent
In the second step, the hydrogen ion is pulled out of the ring by the HSO4- ion acting as a Brønsted base. As in the bromination reaction, the restoration of the delocalization of the pi-electrons facilitates the removal of the hydrogen ion as H+
electrophiles for benzene
donate electrons into its delocalized molecular orbitals. Each of these electron-donating substituents has an electronegative atom with at least one lone pair of electrons attached directly to the aromatic ring.
electrophilic substitution of benzene is much faster when an electron-donating substituent is present.
eg: the nitration of phenol, C6H5OH, proceeds so quickly that it requires no catalyst.
why does phenol react much faster than benzene?
An electrophile is attracted to regions of high electron density. Therefore, to account for the fast reaction of phenol, the electron density must be greater in the ring when the electrondonating O-H substituent is present.
Why is the meta position such an unattractive location for substitution
electron density must be relatively high at the ortho and para positions, higher concentration of electrons in the ring in phenol, especially at the ortho and para positions, than in benzene, largely because the O atom has lone pairs of electrons that can participate in pi-bonding with the carbon atoms
an atom with a lone pair of electrons next to the ring, and all accelerate reaction.
Are there substituents that can slow down the electrophilic substitution of benzene?
substitute a hydrogen atom with a highly electronegative atom or group that can withdraw some of the electron density from the benzene ring, COOH
the nitration of benzoic acid is much slower than nitration of benzene
The electronegative O atoms of the carboxylic acid group withdraw electrons from the whole ring, thereby reducing its overall electron density. resonance preferen-ially removes electrons from the ortho and para positions.
As a result of both effects, the reaction rate is lowered, especially at the ortho and para positions, and the meta position is left as the most likely place for attack.
substituents DONT have a lone pair of electrons on the atom next to the ring.