3.1.4.2 - Enzymes
0.0(0)
Card Sorting
1/21
Last updated 11:22 AM on 11/21/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
1
New cards
enzyme
globular proteins
biological catalyst speeding up the rate of reaction without being used up by lowering the activation energy - lowers the temperature, letting rate of reaction occur faster
biological catalyst speeding up the rate of reaction without being used up by lowering the activation energy - lowers the temperature, letting rate of reaction occur faster
2
New cards
enzyme action
can be intracellular or extracellular
3
New cards
activation energy
minimum amount of energy required to start a reaction
4
New cards
active site
has a complementary shape to the substrate, where chemical reactions occur
5
New cards
substrate
substance that binds to the active site of enzymes and is acted upon
6
New cards
lock and key theory
each enzyme is specific, complementary shaped substrates and active sites of enzymes bind together forming an enzyme-substrate complex, substrate bonds are stressed, breaking the bonds which change the shape and lowers the activation energy, the products leave the active site as they are no longer complementary to the active site
7
New cards
induced fit
substrate is not completely complementary to the active site, active site of enzyme changes shape as the substrate binds, forming an enzyme-substrate complex, bonds in substrate are stressed and broken, lowering activation energy and forms products, products are not complementary to the active site and leaves
8
New cards
enzyme properties
related to tertiary structure - determines active site, it is specific and only catalyses one reaction
9
New cards
anabolic pathway
requires energy and is used to build up large molecules from smaller molecules
10
New cards
catabolic pathway
releases energy and used to break down large molecules into smaller molecules
11
New cards
factors affecting enzymes
ph, temperature, substrate concentration, enzyme concentration
12
New cards
measuring rate of reaction
how fast the product is made, how fast the substrate is broken down
13
New cards
how fast the product is made
measure amount of end product present at different times during the experiment
14
New cards
how fast the substrate is broken down
measure amount of substrate molecules left at different times during the experiment
15
New cards
temperature
high temperature = increases kinetic energy of molecules = more successful collisions between enzymes and substrates, forming more products, increasing the rate of reaction
if temperature becomes too high, the bonds in enzymes starts to break, resulting in a change of the shape of the active site, causing it to denature, the enzyme can no longer function and it is irreversible
if temperature becomes too high, the bonds in enzymes starts to break, resulting in a change of the shape of the active site, causing it to denature, the enzyme can no longer function and it is irreversible
16
New cards
ph
measure of the concentration ratio of hydrogen ions to hydroxide ions
17
New cards
ph effect
enzymes have different optimum pHs, changes in pH affects the charges of amino acids, extreme changes in pH cause ionic bonds to break, tertiary structure of protein changes and the active site is no longer complementary to the substrate
acidic conditions -- increase of hydrogen ions
alkaline conditions - increase of OH ions
acidic conditions -- increase of hydrogen ions
alkaline conditions - increase of OH ions
18
New cards
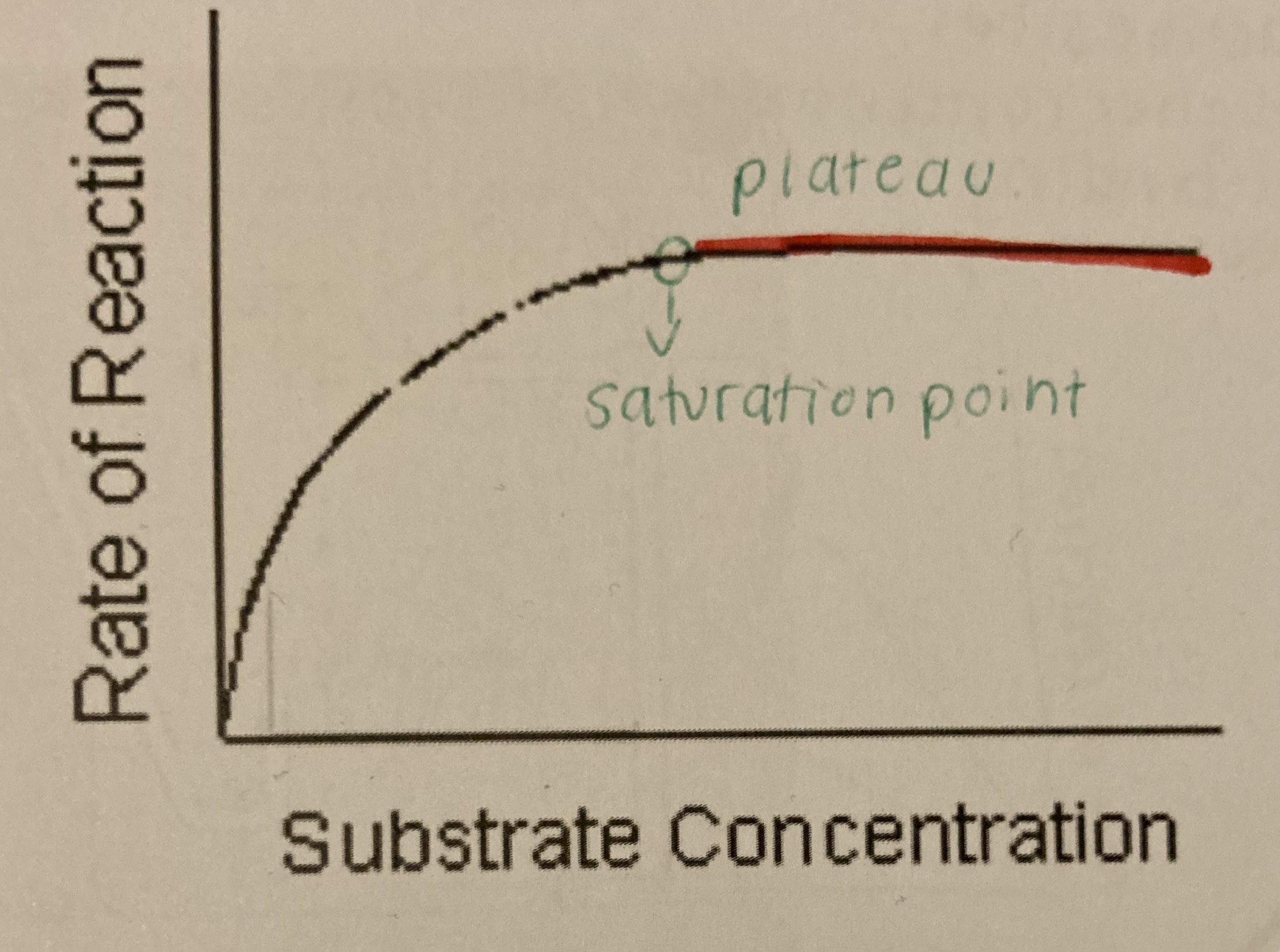
substrate concentration
increasing substrate concentration increases the rate of reaction
low substrate concentration = enzymes have limited amounts of substrates to collide with, the active sites are not working at their full capacity
more substrates = active sites are becoming filled up and are working as fast as they can until it reaches its saturation point and the excess substrate no longer effects the rate of reaction as the active sites are all occupied
low substrate concentration = enzymes have limited amounts of substrates to collide with, the active sites are not working at their full capacity
more substrates = active sites are becoming filled up and are working as fast as they can until it reaches its saturation point and the excess substrate no longer effects the rate of reaction as the active sites are all occupied
19
New cards
enzyme concentration
more enzymes = substrates more likely to collide with an enzyme to form an enzyme-substrate complex
increase enzyme concentration = increase rate of reaction
enzyme concentration no longer effects the rate of reaction when there is a limit to the amount of substrates, enzymes become excess
increase enzyme concentration = increase rate of reaction
enzyme concentration no longer effects the rate of reaction when there is a limit to the amount of substrates, enzymes become excess
20
New cards
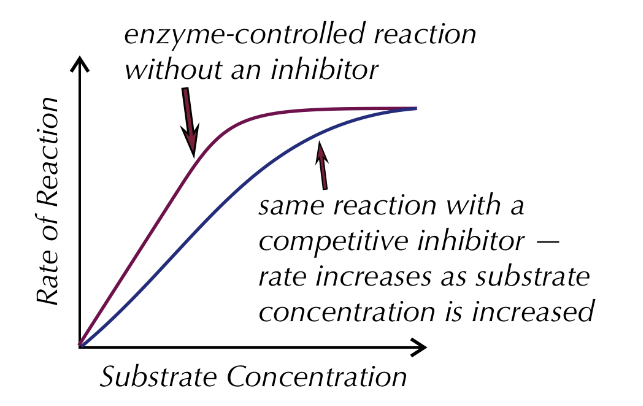
competitive inhibitors
similar shape to substrate, they compete with the substrates to fit and bind the active sites of enzymes, blocking the active site so substrates can't fit in and this inhibits the reaction
high concentration of inhibitors = take up most of the active sites, substrates are less likely to bind to the active sites, only small amounts can
higher concentration of substrate = higher chance of binding to active sites, increasing rate of reaction up to the saturation point
high concentration of inhibitors = take up most of the active sites, substrates are less likely to bind to the active sites, only small amounts can
higher concentration of substrate = higher chance of binding to active sites, increasing rate of reaction up to the saturation point
21
New cards
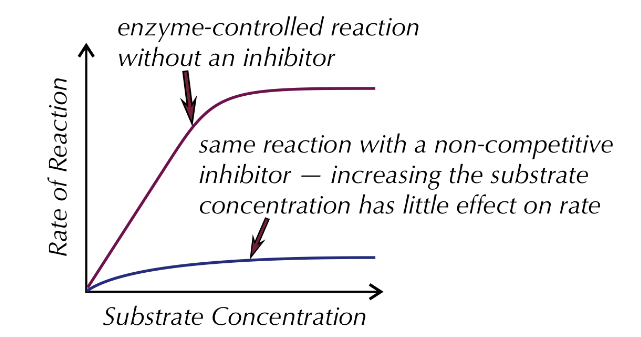
non-comptitive inhibitors
bind to the allosteric site of an enzyme, causing the active site to change, substrates can no longer bind to it as they are not complementary to each other, the enzyme loses its function, it is irreversible
do not compete with substrates as they are different shapes
increase substrate concentration does not effect the enzyme activity as it is inhibited
do not compete with substrates as they are different shapes
increase substrate concentration does not effect the enzyme activity as it is inhibited
22
New cards
allosteric site
part of enzyme that is not the active site