13. Stem Cells and Cell Differentiation
1/46
Earn XP
Description and Tags
exam 4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Is it ok to use hESCs for stem cell therapy (in religious view)?
some Christian denominations oppose research because it requires the destruction of a human embryo
which they consider to be a human life, while others may find its use acceptable
Judaism and Islam generally support stem cell research and therapy based on the principle of preserving life, particularly for treatments like those derived from adult or umbilical cord blood
Multiple uses for Stem Cells
Increased Understanding of How Diseases Develop
Cure diseases
Test New Drugs for Safety
Generate New Stem Cells to Replace or Aid Diseased or Damaged Organs
Research How Certain Cells (e.g. cancer stem cells) develop into
CancerRegenerative Medicine Applications
Fix Genetic Diseases in the future
Tissue Engineering – Organs on a Chip
Clean Meat Industry
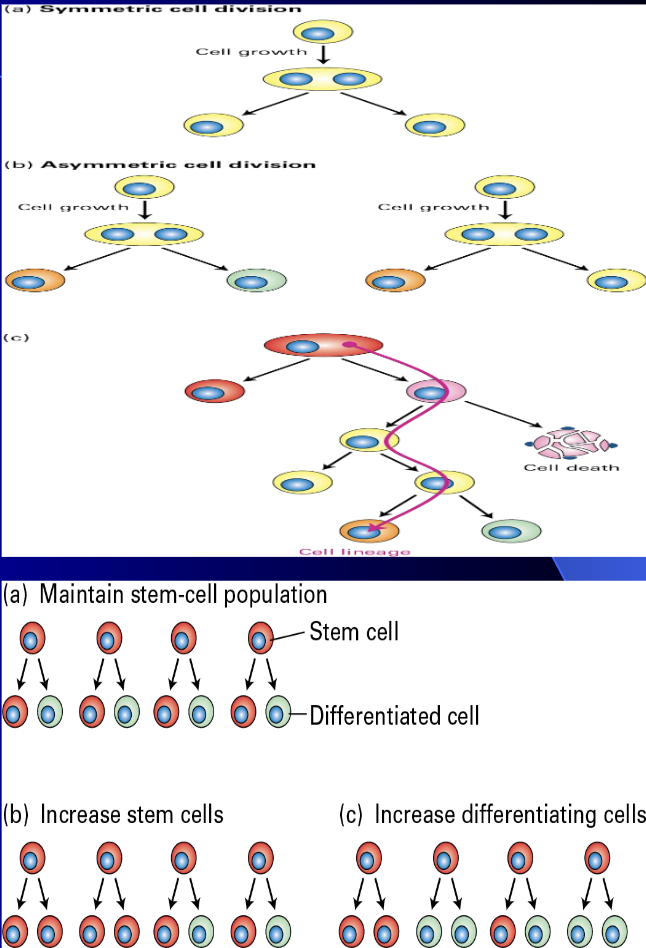
Stem Cell
A cell that can renew (divide) or differentiate
Above controlled by
stem cell “niche”Number of doublings influenced by source and type
hESCs and iPSCs are immortal, adult sourced (i.e. ad-MSCs) 100 to 200+ doublings (approx.) – more than a typical somatic cell that is regulated by the Hayflick Limit
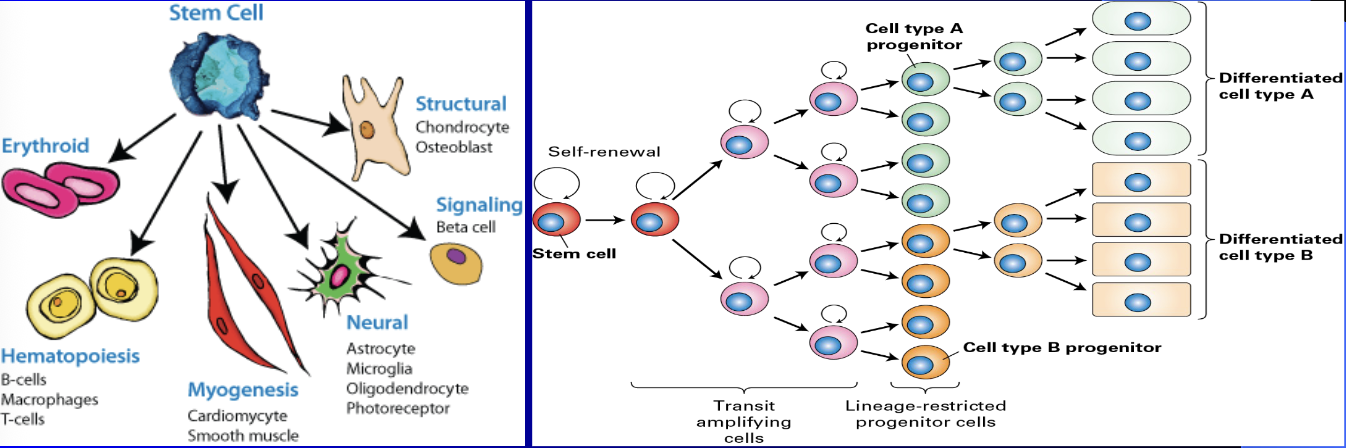
Adult Stem Cells
Most popular are adipose (fat) derived mesenchymal stem cells (adMSCs) now as of 11/20/2025 in more than 3000 stem cell therapy trials globally registered with World Health Organization
Fetal Stem Cells
Amniotic, umbilical cord, placenta
Embryonic Stem Cells
hESCs and hPSCs with hESCs in US clinical trials as of 2010
powerful, undifferentiated cells from a 3-5 day old embryo (blastocyst) that can become any cell type in the body (pluripotent)
Induced Pluripotent Stem Cells (iPSCs)
are not in clinical trials in US but patients being treated in Japan and Australia and elsewhere with 116 clinical trials as of 11/20/2025
Differentiation
Cell becomes more specialized such as a fibroblast or hepatocyte
But differentiation can be partial or full so critical and accepted molecular metrics need to be in place to compare, for instance, one iPSC generated hepatocyte to another iPSC generated hepatocyte. RNA-seq is one metric
Some stem cells have “restricted lineage” and are often called “progenitor” cells because they are limited to only one or two types of cells, while others are totipotent
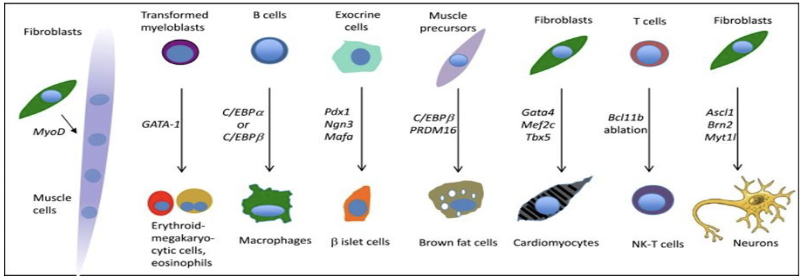
Transdifferentiation (Direct Reprogramming)
Ability of a differentiated cell to become another type of differentiated cell without going through an embryonic step (e.g. unlike iPSCs)
First done experimentally in 1987 but several cells have been generated since that time
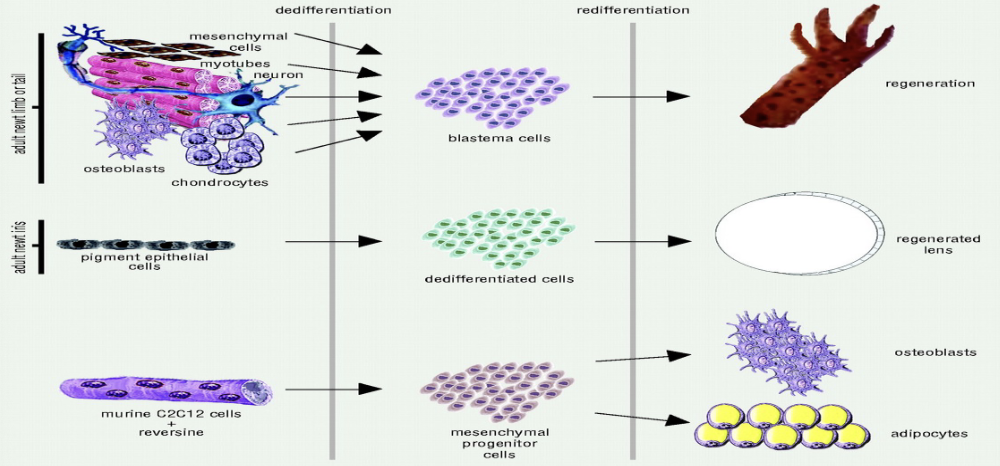
Dedifferentiation and Redifferentiation
Ability of a cell to become more embryonic-like and differentiate into another cell type in vivo
Chemicals like “reversine” can induce de-differentiation
ex: Eastern Red Spotted Newt
Stem Cell Niche
Also called the stem cell microenvironment
Critical to controlling cell division vs differentiation
Complex and includes
Neighboring cells, extracellular matrix, local growth factors (FGF, others), physical environment (pH, oxygen tension, pressure)

Potency
Totipotent
All cell types
Highest level of “stemness”
Pluripotent
Many cell types
Restricted stemness
Multipotent
Several cell types
Stemness even more restricted
Unipotent
One cell type only

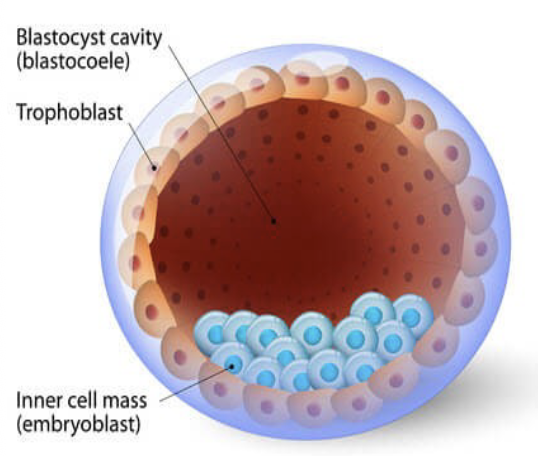
Blastocyst
late pre-implantation stage embryo
hESCs originate from inner cell mass
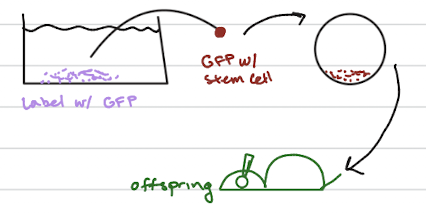
Chimera Test Can Determine if a Stem Cell is Totipotent in vivo
Legal with mice but not with humans
Thus, we can never prove that any human stem cell derived or isolated in
the lab is truly totipotent in vivo in humansOnly true test of totipotency of a candidate stem cell
Label test stem cell with GFP (green fluorescent protein)
Implant GFP-labeled test stem cell in blastocyst and then implant chimeric embryo in surrogate mother
Now track that GFP labeled stem cell in all tissues and organs of newborn
mESC (mouse derived) are totipotent but can’t say the same for hESC
How can you tell if a candidate stem cells is Totipotent
chimera test
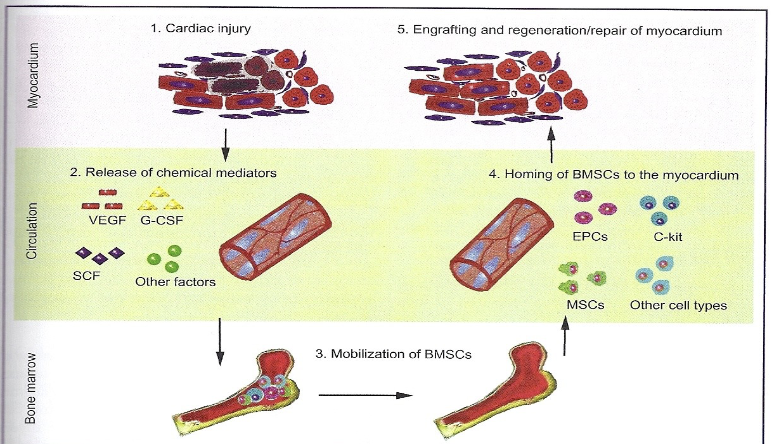
Biodistribution and Homing
Ability of stem cells to find “home” – its targeted tissue
Damaged or compromised tissue releases factors that causes
endogenous MSCs to home to damaged siteOccurs in vivo: Transplanted XX hearts in XY patients have XY cardiomyocytes upon autopsy (10%) – a clear demonstration of endogenous stem cell homing and repair
Shinya Yamanaka
Induced Pluripotent Stem Cells (iPSCs)
won the 2012 Nobel Prize
STAP (Stimulus Triggered Acquisition of
Pluripotency)
a method reported in 2014 where mature cells were supposedly reverted to a pluripotent or even totipotent stem cell state by simply exposing them to mild stress
The study, claimed these STAP cells could contribute to all tissues and the placenta in a chimeric mouse, was later retracted and deemed fraudulent due to issues with the data and findings
The accompanying poll shows the public's initial divided belief in the existence of STAP cells prior to the retraction
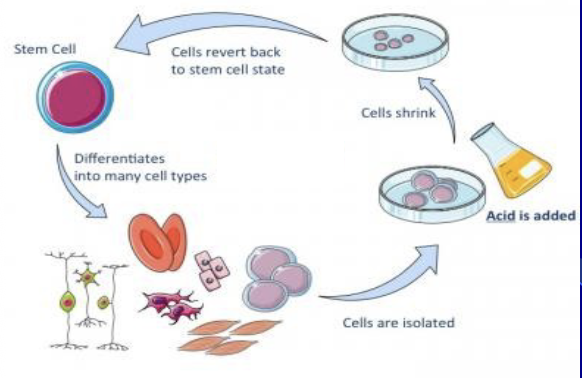
Fusogenic
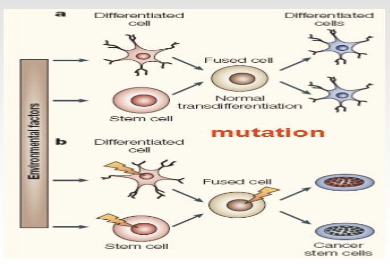
Problem with stem cells
They can spontaneously fuse with each other forming a tetraploid cell (could generate cancer stem cells)
When injected into patients mechanical stress can cause fusion
Bioethics
the norms of conduct
relative terms and country dependent
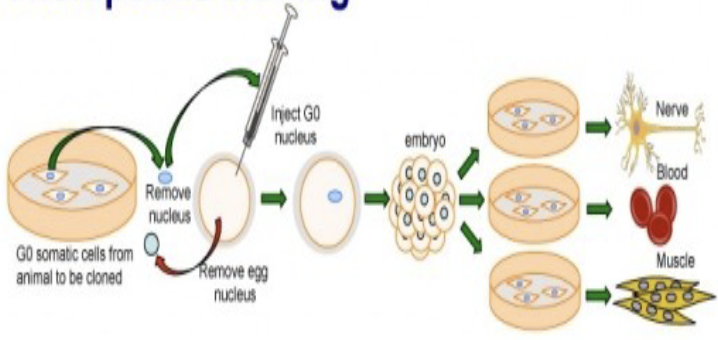
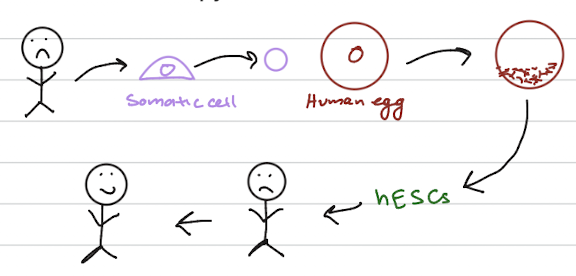
Therapeutic cloning
the production of embryonic stem cells for the use in replacing or repairing damaged tissues or organs
achieved by transferring a diploid nucleus from a body cell into an egg whose nucleus has been removed
creating embryo develops under laboratory conditions
responsible for creating embryonic stem cells to treat diseases such as diabetes and Alzheimer’s disease
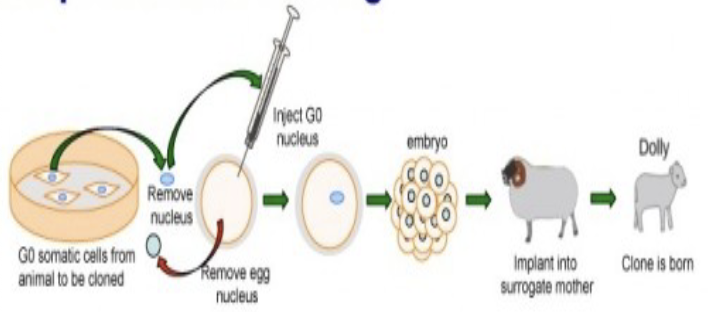
Reproductive cloning
the deliberate production of genetically identical individuals; each newly produced individual is a clone of the original
creating embryo develops under uterine conditions
important for harvesting stem cells that can be used to study embryonic development
SCID (Severe Combined Immuno
Deficiency) Mice
Have no B and T cells and thus have a
compromised immune systemAre used for determining if an injected candidate stem cell can differentiate in vivo into a multitude of tissue and cell types in vivo
Are also used to determine if a candidate human cancer cell can generate tumors in vivo

Three Ways to Generate Stem Cells in the Laboratory
Somatic Cell Nuclear Transfer (SCNT)
Parthenogenesis (hPSCs)
Induced Pluripotent Stem Cells (iPSCs)
SCNT (Somatic Cell Nuclear Transfer)
Could be used for autologous or allogeneic stem cell transplants
No US federal laws ban therapeutic or reproductive cloning research but some states forbid it. But not allowed clinically in the US
But is it ethical? A “human embryo” is being created
hPSCs (human parthenogenetic stem cells)
a type of human pluripotent stem cell derived from an unfertilized egg that has been chemically activated, rather than through fertilization
share many properties with human embryonic stem cells (hESCs), such as the ability to self-renew and differentiate into all three germ layers,
potential advantages for cell-based therapies like reduced risk of immune rejection
Benefits of hPSCs for therapeutic cloning
Only 200 to 300 eggs would be required to generated hPSCs that could match anyone in the world
Limitations of hPSCs for therapeutic cloning
All alleles will be homozygous because of no sperm thus chance of phenotypic expression of a mutation is high compared to heterozygote
Not FDA approved in US
Is it ethical to create a human embryo?
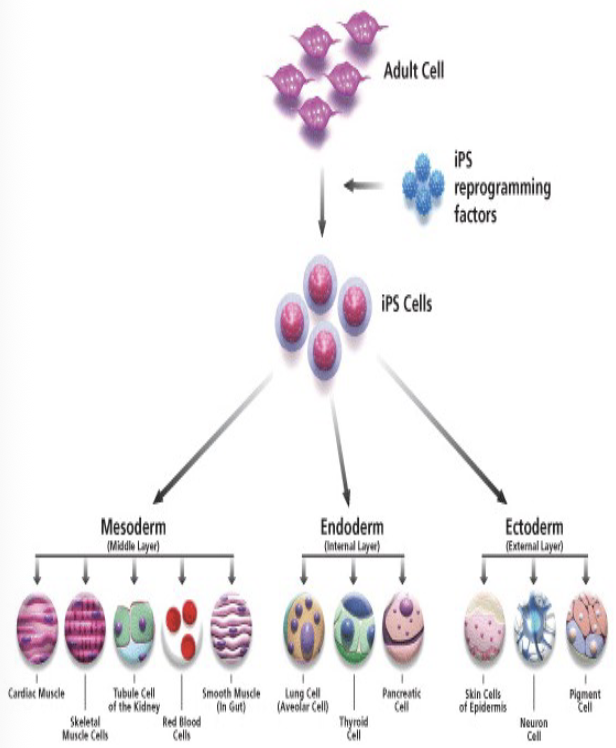
iPSCs (Induced pluripoint stem cells)
Sir Ian Wilmut cloned Dolly the sheep in 1996 and John Gurdon's was the first to work with frogs
No “human embryo” created as in SCNT and Parthenogenesis
Can be autologous or allogeneic
But potential for teratocarcinomas
More pluripotent than fat (adipose)-derived adult mesenchymal stem
cells and easier to procure

RT-PCR (Real Time Reverse Transcription Polymerase Chain Reaction)
a sensitive and fast test used for detecting the presence of specific genetic materials within a sample
Is a teratoma generated by iPSC injection?
generated by injecting induced pluripotent stem cells (iPSCs) into immunodeficient mice
standard method to confirm the pluripotency of iPSCs

Teratocarcinoma
A malignant teratoma that
originates from embryonic cells or stem cellssymptoms like a painless, firm lump in the scrotum or, if they grow large, pain and swelling in the abdomen, chest pain, or shortness of breath
Treatment typically involves a combination of surgical removal, chemotherapy, and radiation therapy
Can iPSCs and hESCs do this too? Are iPSCs
in clinical trials like hESCs?
not yet reached the clinical trial stage in the United States
(FDA) has cleared multiple Investigational New Drug (IND) applications for iPSC-derived therapies to enter trials
Promise of iPSCs
Basic research on differentiation
Can make patient specific cells of
individuals carrying genetic defects –useful for drug developmentSource of cells in the future for stem cell therapy
Not yet FDA approved but Cynata Therapeutics (Australia) just completed first clinical trials in UK using iPSCs.
Have proven very useful in tissue
engineering organoids et..
Parthenogenesis
Can match to a world population – only 300 eggs required (?)
But all alleles are homozygous, not heterozygous
Allogeneic, not autologous like SCNT unless female donated egg
But is it ethical? A “human embryo” is being created
Tumorigenicity
Stem cells have long telomeres and can divide many more times
than normal cells. (Telomeres = “mitotic clock”)Propensity to form tumors such as teratocarcinomas
One clinical trial started in Japan overseen by the RIKEN Institute
(later) was stopped after only one patient due to this concern
Immunogenicity
Propensity to trigger immune response
The more frequent the stem cell injections the higher the chance of
immune rejection complications that could include anaphylaxisAutologous as well as allogeneic can launch an immune response
Inappropriate differentiation
Risk of stem cells differentiating into cells that were not intended
and not native to target organ
ex: A woman injected with human mesenchymal stem cells (MSCs) near her eyes ended up with bone tissue growing inside her eyelids
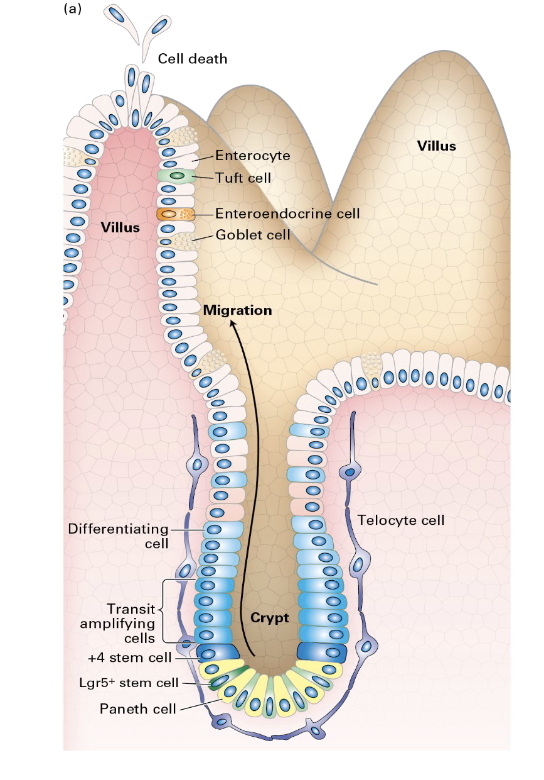
Regeneration of the Intestinal Epithelium
a rapid process driven by intestinal stem cells (ISCs) located in the crypts, which replenish the cells of the villi every 5–7 days
involves ISCs differentiating into progenitor cells, which then become mature cell types
The regenerated cells move up the villi, where they perform their function before undergoing programmed cell death at the tips
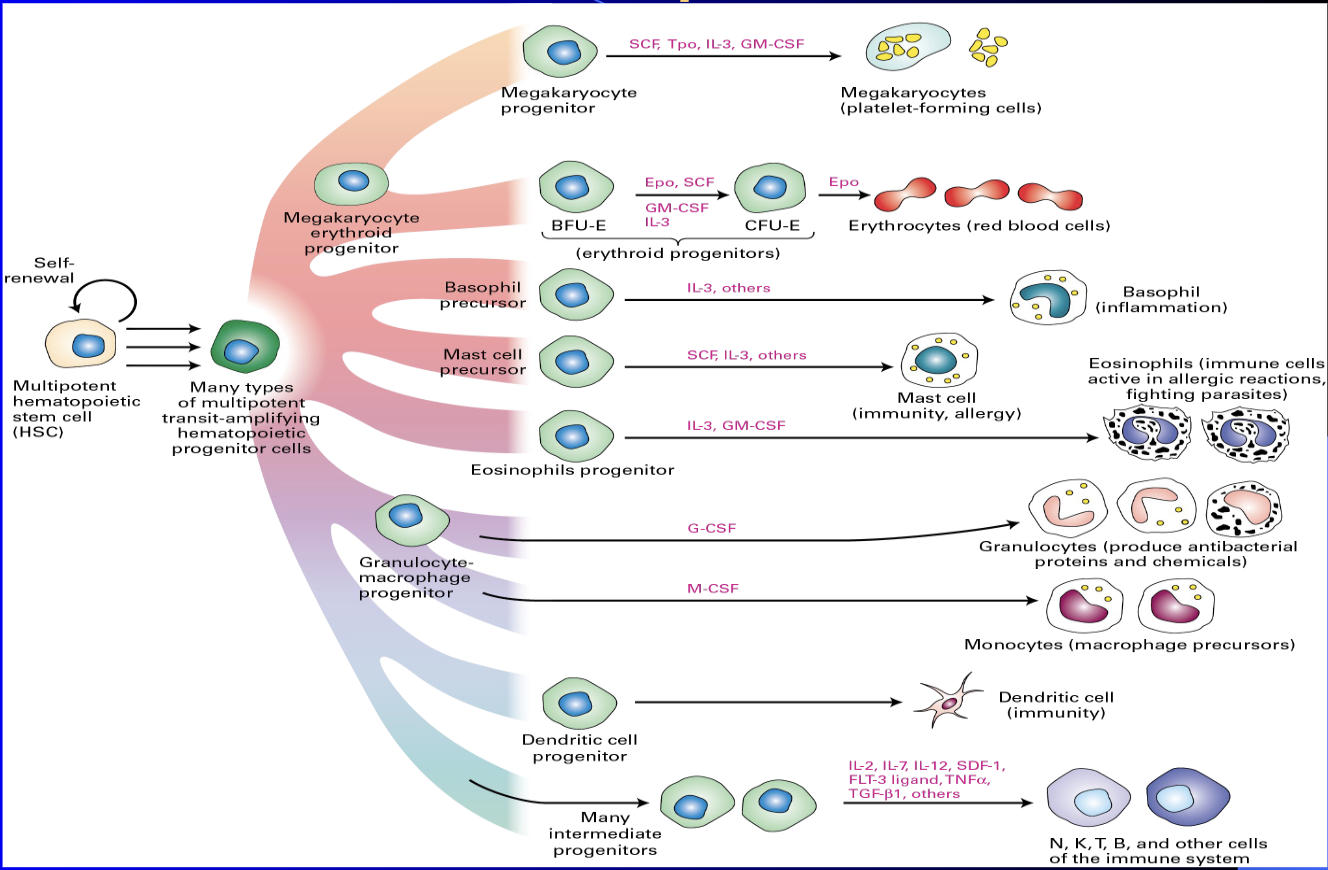
Hematopoiesis
process of creating all types of blood cells from hematopoietic stem cells (HSCs)
crucial for maintaining blood cell homeostasis, as it involves the HSC niche
The production is tightly regulated by growth factors and cytokines, such as stem cell factor (SCF) and interleukins, and is essential for the body's immune response and oxygen transport
Cord Blood
“Blood Replacement and Stem Cell Therapy"
contains Hematopoietic Stem Cells and Mesenchymal Stem Cells, which can be used for blood replacement and regenerative therapies
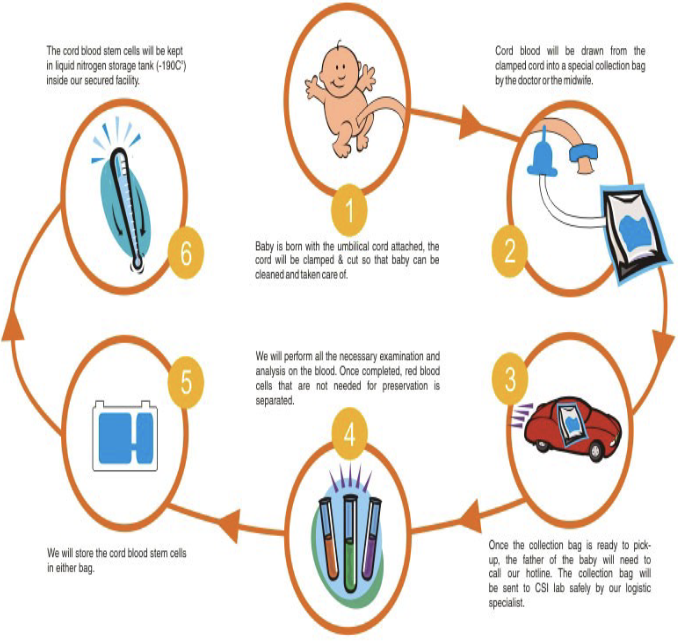
Cord Blood - Private
incorporated as a “for profit” organization
Donors pay an initial fee and a maintenance fee
Cells not available to the public
Better if there is a genetic disease in the family and multiple members require the cells
Cord Blood - Public
Incorporated as a “not for profit” organization
Available to the public through the National Marrow Donor Program through which cord blood is matched
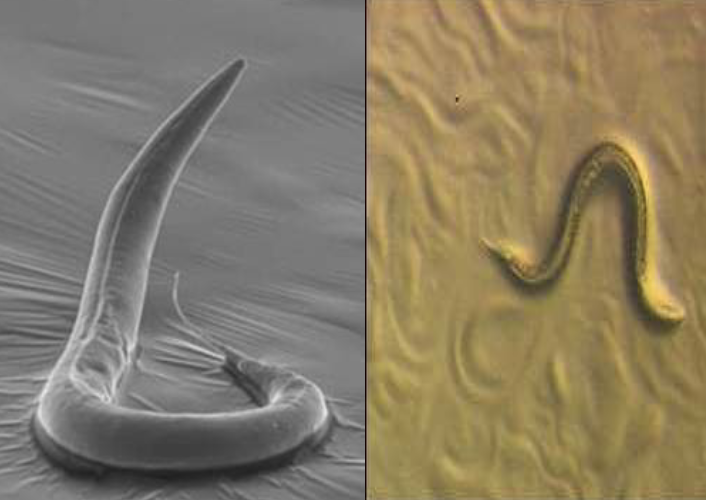
Benefits of C.elegans
Easy to grow on agar plates and is a non-pathogenic roundworm
Comprised of a limited number of cells (about 1000)
Translucent – can optically section through organism
Stable mutants of ___ are available for study
All cells have been coded with a cell specific letter/number code
Cell division/differentiation patterns can be predicted and always follow the same pattern
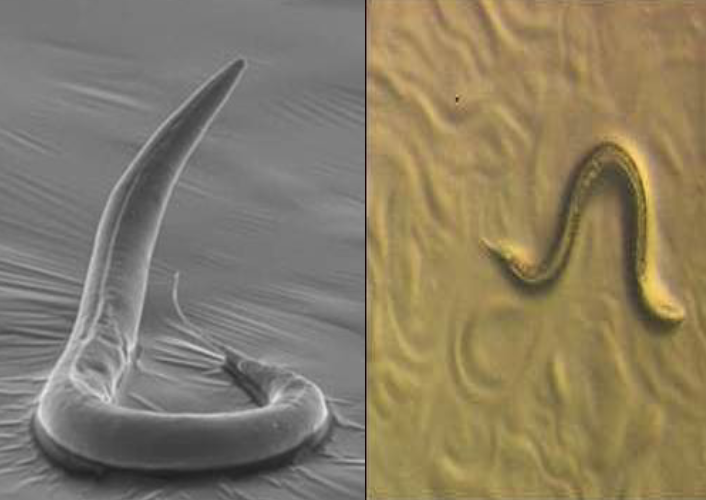
Contributions of C.elegans
RNAi was first discovered in C. elegans (Nobel Prize in 2006)
The apoptotic genes were first identified in C. elegans (Robert Horvitz won the 2002 Nobel Prize for Physiology or Medicine for his work on apoptosis in C. elegans)
Many genes like the apoptotic genes have mammalian homologs
First microRNA (miRNA) discovered (Nobel Prize in October, 2024, to Victor Ambros and Gary Ruvkun)
When and How is Organismic
Polarity Established?
during early development through a process of symmetry breaking,
guided by cues like secreted proteins, and then maintained by internal cellular mechanisms
external or internal cues break the initial symmetry, followed by the signaling of membrane-associated receptors, the recruitment and reorganization of the cytoskeleton, and the polarized localization of proteins
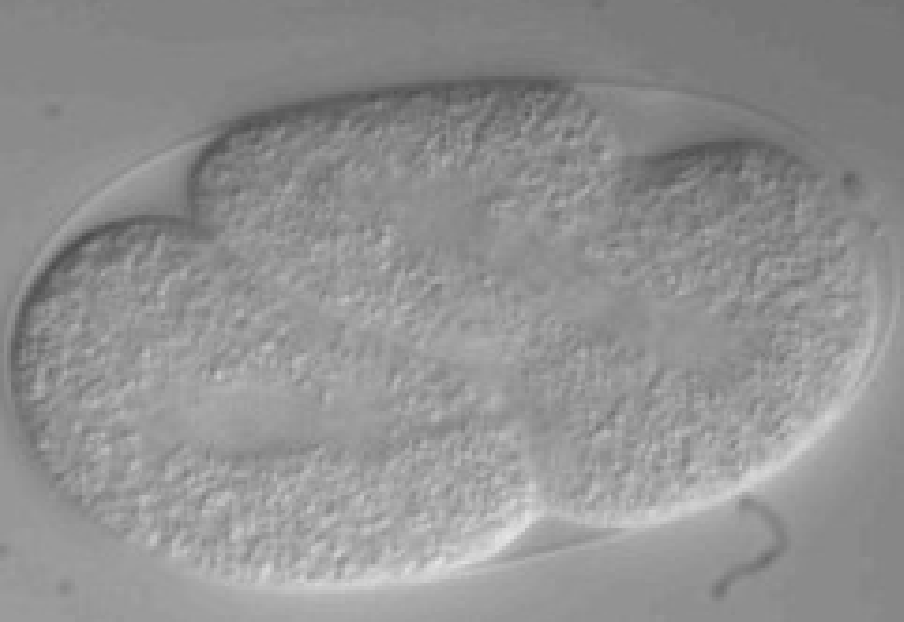
C. elegans - par proteins establish polarity
by forming two opposing cortical domains—an anterior domain with PAR-3, PAR-6, and PKC-3, and a posterior domain with PAR-1 and PAR-2
initiated after fertilization by the sperm centrosome, which causes a contraction of the actomyosin network that pushes the anterior PARs to the anterior cortex while an independent feedback loop maintains the posterior PARs at the posterior
established polarity ensures the first embryonic cleavage is asymmetric, leading to daughter cells with different fates and division patterns.