BIOL21351 L10&15 lol
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
52 Terms
What cells are abundant in chronic inflammation?
Predominantly monocytes/ macrophages and lymphocytes
What causes chronic inflammation?
- Persistent infection
- Hypersensitivity
- Prolonged exposure to toxins
- Immune-mediated (e.g. antibodies against the myelin sheath)
Give examples of toxic agents that can lead to chronic inflammation
Exogenous e.g. asbestos
Endogenous e.g. uric acid crystals
Where do macrophages come from?
Arise from monocytes in the blood, but differentiate into macrophages when they migrate to tissue
Name some diseases associateed with inflammation
Atherosclerosis
How are acute and chronic inflammation similar?
Many of the same cells, receptors and mediators are involved in both
What happens when the adaptive immune system is involved?
Tissue damage occurs, leading to the cycle of inflammatory events
Give possible treatments to reduce circulating cholesterol
Diet, exercise, stop smoking, reduce alcohol
- Lipid-lowering drugs like fibric acid derivatives (bezafibrrate) and statins
- PPAR activators
Outline the mechanism of lipid lowering drugs (2)
Bezafibrate decreases VLDL and triglycerides
Statins inhibit HMG-CoA reductase (the rate limiting step in cholesterol synthesis)
How do statins treat atherosclerosis?
- They inhibit HMG-CoA (preventing cholesterol synthesis
- They suppress platelet activation
- Plaque stabilisation
- Reduced cytokine production
- Upregulation of IL-10
Mechanism of ApoE?
Inhibits oxidation of lipoproteins, VSM cell proliferation and migration and inhibits platelet aggregation
Results of CANOTS study
- Canakinumab prevents adverse cardia events
- Reduces CRP (dose-dependent)
- Associated with increased risk of fatal infection by sepsis
Mechanism and role of colchicine
Inhibits assembly of the NLRP3 inflammasome and therefore IL-1β secretion, so reduces inflammation
Source of cholesterol
- From diet AND
- Produced by liver
What are chylomicrons?
tiny fatty droplets composed of triglycerides, small amounts of phospholipids, cholesterol, free fatty acids, and some protein.
What is the role of chylomicrons?
Transport cholesterol from the small intestine to the body
Where are chylomicrons synthesised?
In the GI tract
PPAR activation leads to...
Decreased expression of adhesion molecules, foam cell formation and other effects that decrease inflammation and atherosclerosis
List the cells in both chronic and acute inflammation and those in chronic only
Both : Plasma cells, eosinophils and mast cells
Chronic only : Lymphocytes
What is the difference in action of the chronic compared to the acute inflammatory process?
Chronic involves lymphocytes and the adaptive immune system, whereas acute involves the innate immune system.
Risk factors for atherosclerosis that involve the inflammatory response
Increased CRP, interleukins and coagulation factors
What is a fibrous cap?
Result of smooth muscle cell apoptosis during atherosclerosis.
Fibrous cap forms over lipid-rich accumulation.
When fibrous cap ruptures, endothelium is exposed to circulating platelets and coagulants -> acute thrombus forms
What is endothelial dysfunction?
-Change in endothelial cells leading to malfunction
-Implicated in thrombus formation, atherosclerosis & other disorders (Ex. HTN)
-May be rapid or slow in onset & reversibility
Under normal conditions, what does endothelium control?
- The vascular tone
- The balance between thrombosis and fibrinolysis
- Regulates the recruitment of inflammatory cells
How does a plaque develop?
damage to endothelial cells (due to cholesterol, high blood pressures, or LDL)
this damage causes inflammation and hangs artery lining
Steps to plaque development
1. Endothelial Dysfunction
2. Lipid accumulation
3. Inflammatory response
4. Foam cell formation —> Plaque
How do foam cells form?
Oxidised LDL trapped in macrophages forms foam cells
What makes up a tumour (carcinoma)?
Neoplastic cells and non-neoplastic stroma
What can limit cancer growth?
Lack of oxygen (hypoxia) and metabolic waste products)
What is the role of stroma?
Contributes to tumour progression
How does hypoxia alter gene expression?
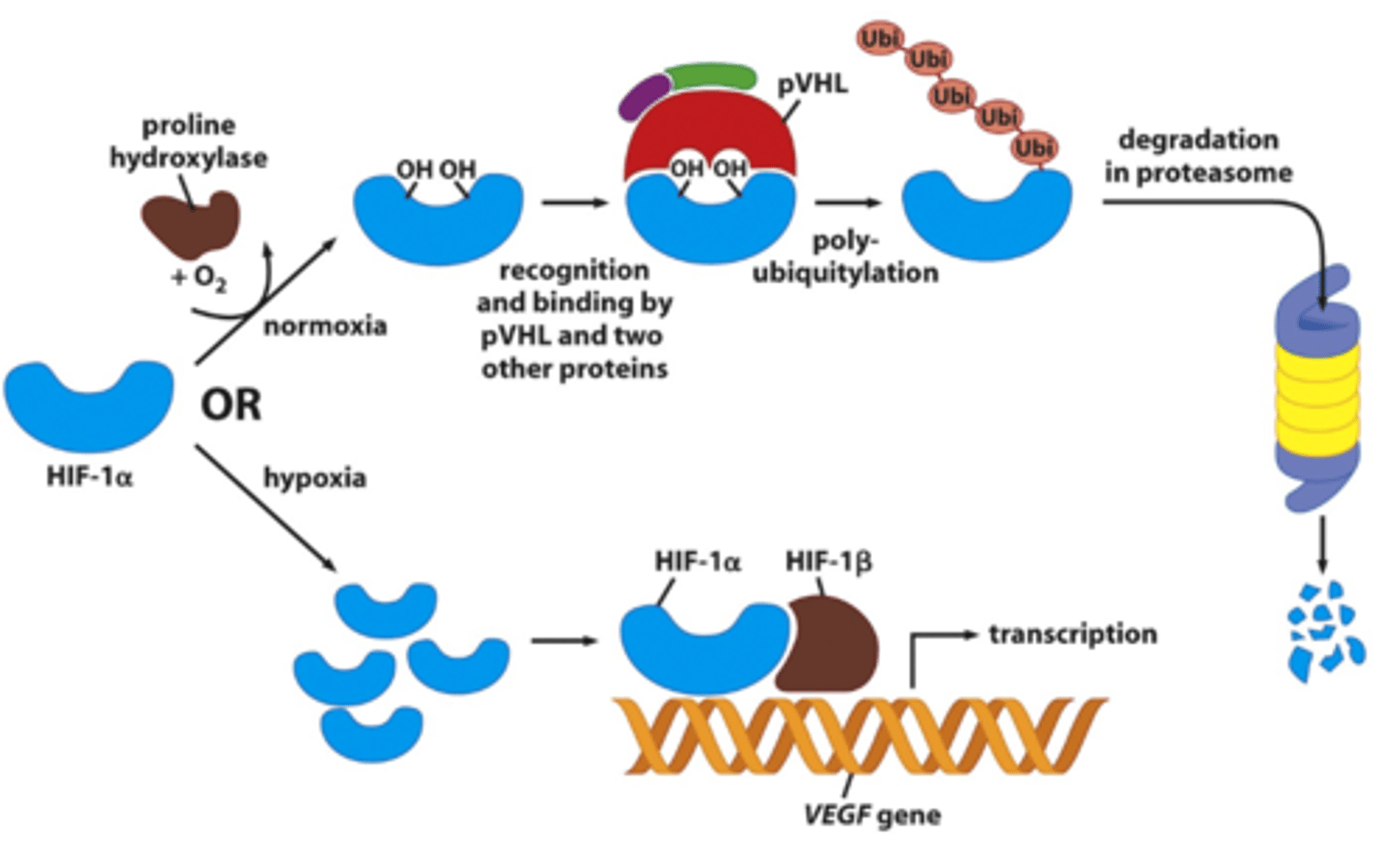
Mechanism of angiogenesis
1. Pericytes detach
2. BM and ECM degraded by MMPs
3. Endothelial cells migrate towards the angiogenic stimulus
4. Endothelial cells proliferate in a migration column to tumour
5. Endothelial cells adhere to each other and a blood vessel forms

What is stroma, with regards to tumours?
The surrounding tissue of tumours that contains ECM, fibroblasts and immune cells
What is neovascularisation?
natural formation of new blood vessels that is required for tumour growth
Why is tumour vasculature chaotic and leaky?
Fenestrations (small holes) in endothelial cells. This raised hydrostatic pressure within the tumour reduces distribution of chemotherapy
How is neovascularisation achieved?
Through the angiogenic switch
What is the angiogenic switch?
An increase of activity in angiogenesis activators (e.g. VEGF) and a reduction of inhibitors (e.g. endostatin) that results in neovascularisation (for tumour growth)
Give examples of angiogenesis inhibitors and activators
Inhibitors: Statins and Thrombosponin-1
Activators: VEGF, FGF, EGF
Different mechanisms that inhibit angiogenesis
- Inhibiting the signalling cascade
- Inhibiting endothelial cells
- Blocking the ability of endothelial cells to break down ECM
Drugs that inhibit the angiogenesis signalling cascade
Drugs that inhibit VEGF, e.g. avastin and SU6668
Drugs that target endothelial cells directly to prevent angiogenesis
Endostatin,
Thalidomide
Drugs that block the break down of ECM
Marimistat - prevents MMP production
Processes that lead to neovascuarisation
- Angiogenesis
- Vasculogenesis
- Vascular mimicry (cancer cells form vessels themselves)
Mechanism of vasculogenesis
New vessels form de novo from circulating endothelial progenitor cells released from the bone marrow
Where do angiogenic factors come from?
Tumour cells, mast cells, macrophages and ECM
Which lysing processes are increased in cancer?
Glycolysis and glutaminolysis
Why do glycolysis and glutaminolysis increase in cancer?
To maintain high levels of anabolic reactions whilst generating sufficient ATP even under hypoxic conditions
Therapeutic targets for cancer growth
Angiogenesis
Altering cellular metabolism
What is the Warnburg effect?
Aerobic glycolysis carried out by cancer cells that leads to an increased uptake of glucose (4 ATP compared to 2 in anaerobic glycolysis)
How does cancer cell metabolism differ from normal cells?
More glutaminolysis
Results in a shift in metabolism to biosynthesis
Produces proteins and nucleic acids from glycolysis and glutaminolysis as well as ATP
What happens in glutaminolysis?
Q —> D/E/A/CO2/Lactate/Pyruvate/Citrate
Also produces carbon and nitrogen
What is the role of glutaminolysis?
Breaks down glutamine to produce components that can be used in respiration to generate energy (citrate, pyruvate —> lactate)