2 - electron structure
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
What is an atomic orbital
Place where electrons are most likely to be around a nucleus
2
New cards
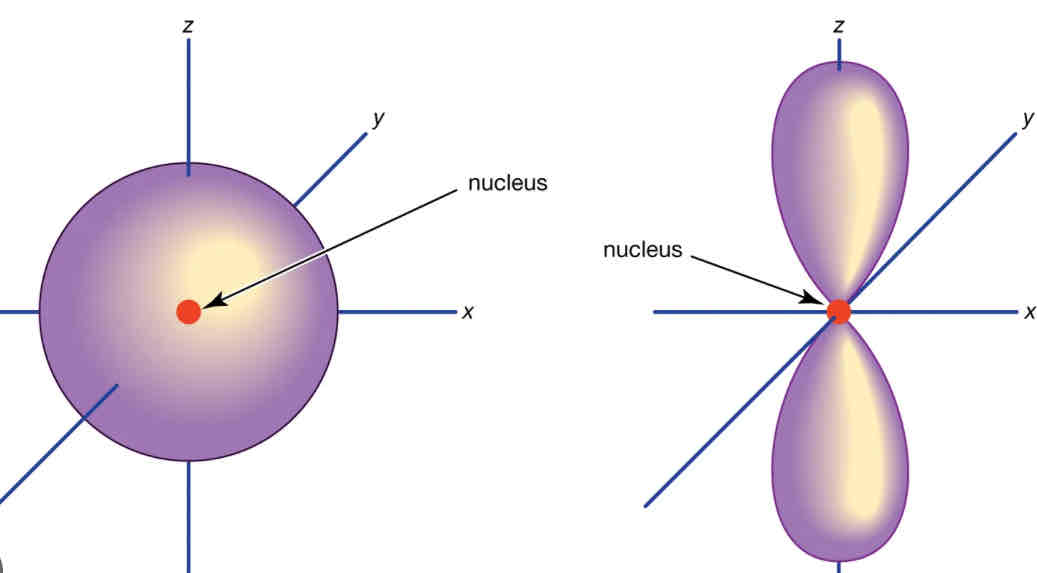
Shape of s/p subshell
S subshell = sphere
P subshell = dumbbell
3
New cards
How many orbitals is s/p/d subshell
S - 1 orbital
P - 3 orbitals
D - 5 orbitals
4
New cards
fff
ebene
5
New cards