gases in the atmosphere
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
what are the four main gases in the atmosphere
nitrogen - 78%
oxygen- 21%
argon 1%
carbon dioxide 0.04%
what are the two methods that measure the proportion of oxygen
iron wool method
bringing phosphorous
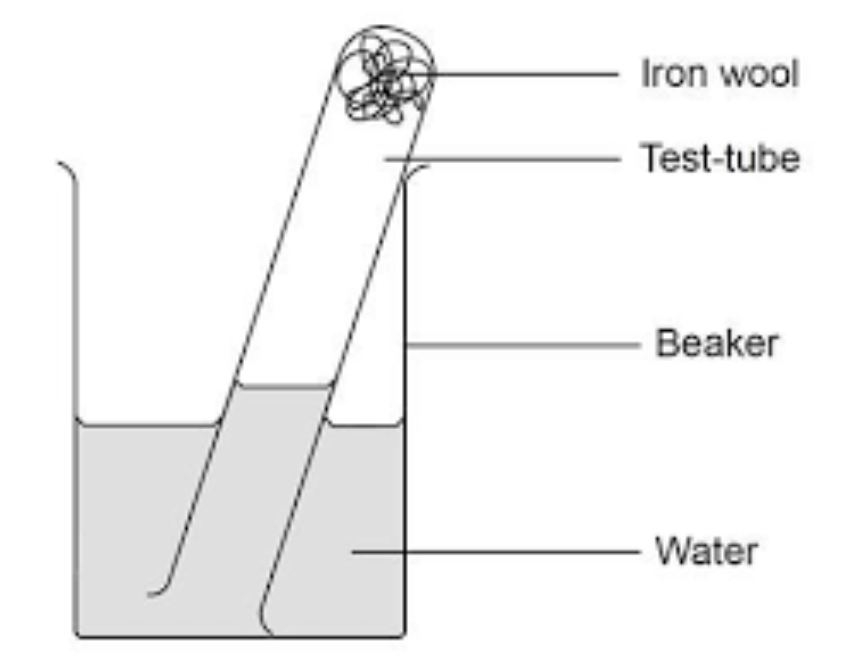
how would you set up the iron wool method
place iron wool in a test tube
invert the test tube in the beaker that has water
leave for several days
what happens is that the iron reacts with the oxygen in the tube, and the water rises to replace the oxygen that has been removed by the iron wool reacting with the oxygen.
Measure the change I the water level
what is the formula to finding the percentage of oxygen
start volume- final volume / start volume x 100
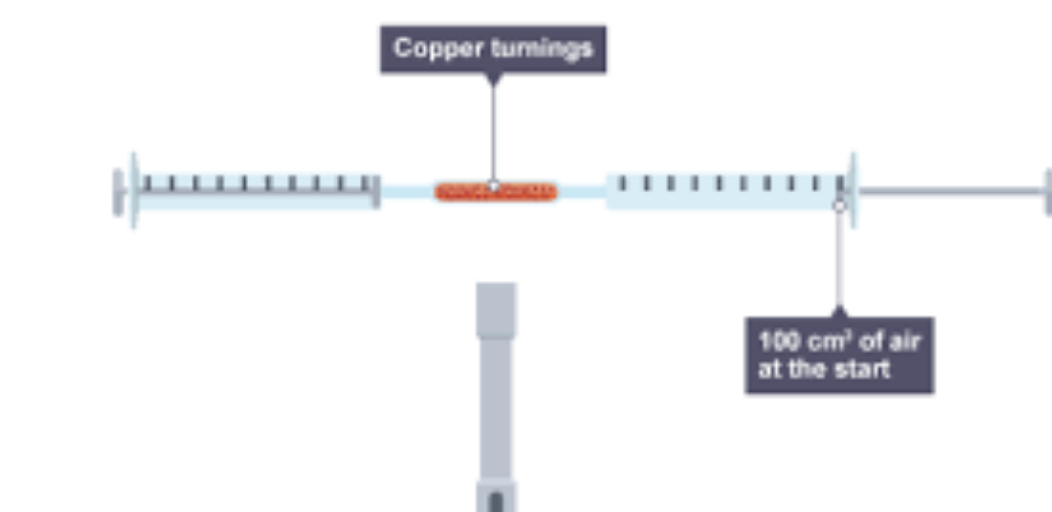
how would you set up for the burning phosphorous method
place phosphorous in a tube
attack the tube with two glass syringe one at each side (make sure one of the glass syringe is extended fully and the other one isn't )
heat the phosphorus using the bunsen burner
use the syringes to pass the air, the some of the oxygen will react with the phosphorous to make phosphorous oxide.
During that the volume of oxygen will decrease.
Then calcualte the percentage of using the formula.
define combustion
chemical reaction where substance reacts with oxygen
what are the three combustion reactions that we have to know
magnesium
bright white light
forms magnesium oxide (white powder)
hydrogen
makes a Loud pop sound
forms water
sulfur
burns with a blue flame
forms sulfur dioxide
define thermal decomposition
breaking down a substance using heat
what is the equation of a metal carbonate when going through thermal decomposition
metal carbonate —> metal oxide + carbon dioxide
what is the thermal decompsotionj reaction of copper carbonate
once it gets heated, it turns into carbon dioxide an copper oxide.
define greenhouse affect
gases that trap the heat making the earth more warmer
why does carbon dioxide cause climate change
becuase the carbon dioxide absorbs the infrared radiation, so more carbon dioxide means more heat in the earths atmosphere
how could the earth increase carbon dioxide levels
burning fossil fuels
deforestation
industries