Unit 2 - Pain Syndromes And Analgesia
1/182
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
183 Terms
What is a noxious stimulus?
A noxious stimulus is one which causes pain
What is the pain scale and why is it used?
The pain scale is used as a way to define a patients pain intensity. It scores pain on a scale of 0-10:
0: No pain
1-4: Mild pain which is more annoying and irritating
4-6: Moderate pain: Pain which can affect daily tasks
7-10: Severe pain: debilitating pain which can affect normal activities
How is pain duration characterised?
Acute: Lasting less than 36 months
Sub-acute/ inflammatory: Pain caused by injury to tissue leading to inflammation
Chronic malignant: This is pain which is caused by central/ peripheral injury lasting more than 36 months
Chronic non-malignant: This is pain which is neuropathic in nature. Long-term.
What are the 3 different types of pain?
Noxious pain - this is pain caused by injury. It is protective in nature and transient
Neuropathic pain - This is pain which occurs in the somatosensory system and tends to be more long-term
Inflammatory -This is due to damage to the tissue, protective to prevent use and prevent further damage
What is referred pain?
Referred pain is pain which is reflected from a different region in which the pain originates, an example is pain radiating down the left arm in response to a heart attack, or ‘brain freeze’ in response to cold sensation hitting the vagus nerve.
What is phantom pain?
Phantom pain is pain which occurs following amputation
Explain how pain is signalled to the brain: How do we feel pain?
Nociception - This is a noxious stimulus felt in the periphery by nociceptors
Pain gating - The nociceptors transmit signals from the periphery to the dorsal horn of the spinal cord. The stimulus is sent up the ascending fibres to the brain
Processing - The signal is processed in the brain and information is sent back down the spinal cord via the descending fibres to different regions of the body.
What are 3 stimulants of pain fibres?
Bradykinin
5-HT3
Histamine
Explain the gate control theory of pain.
The gate control theory states that…
C fibres in the periphery use glutamate receptors which allow for nerve transmission of pain signals. When a sufficient stimuli takes place, the glutamate receptors are activated and allow for an influx of Na+ on the post-synaptic neuron, ultimately leading to depolarisation and an action potential. This allows the pain signals to travel to the dorsal horn of the spinal cord and then to the brain.
Branching off from these C fibres are inhibitory interneurons which, when activated, release GABA (an inhibitory neuron) which prevents the transmission of a signal
When a stimulus is sufficient enough, these inhibitory interneurons will be inhibited to allow for signal transmission.
However, in mild pain, we can activate alpha-delta fibres which run close to the C fibres. The alpha-delta fibres are sensory neurons which, once activated, will allow for activation of the inhibitory interneuron, leading to the release of GABA. This inhibits the pain signal: Hence the saying ‘rub it better’ actually works in mild pain e.g. scatch.
All pain signals are sent to which area of the brain?
Pain signals are sent from the periphery to the spinal cord and then enter the brain regions: the hypothalamus, the amygdala, and the somatic sensory region. From here, all the pain signals are sent to the periaqueductal grey. Note that if we over stimulate the periaqueductal grey then we get no pain output.
How does the treatment of pain vary depending on the region in which it occurs?
Nociception - this is the activation of pain in the periphery and this can be treated with NSAIDs
Pain gating - this occurs in the dorsal horn of the spinal cord and can be targeted with opioids
Pain perception - this occurs in the brain and can be treated with opioids
What are the endogenous ligands called which bind to opioid receptors? What is their role?
dynorphins and enkephalins - have a role in pain perception, motor control, regulation of ANS and neuroendocrine function
Name the different opioid receptors
Kappa, delta and mu receptors
Name the side effects of targeting the opioid receptors
reduced pain perception
euphoria
dependence
respiratory depression
tolerance
nausea
constipation
How is kappa an exception in terms of the side effects it produces?
Kappa is not associated with euphoria
Kappa stimulation can lead to sedation
Name the location of opioid receptors and how this relates to the side effects produced.
The opioid receptors are located throughout the body…
Opioid receptors in the hypothalamus which regulates homeostasis
Opioid receptors in the cough centre of the medulla - hence can be used for suppression of dry cough e.g. coediene linctus
Opioid receptors in the respiratory system of the medulla - can cause respiratory depression
Opioid receptors in the gut - can cause constipation - reduced gut motility
Opioid receptors in the chemo trigger zone -can cause nausea and vomitting
Explain the metabolism of morphine occurs?
The metabolism of morphine results in the production of two metabolites
Morphine-3-glucoronide - Major - inactive
Morphine-6-glucoronide - Minor - active
Morphine-3-glucoronide is inactice and the formation of a glucoronide means it is more water soluble so more easily excreted from kidney
Morphine-6-glucoronide is active but is the minor product
Codiene is a…
prodrug of morphine
Name an opioid full agonist that exists naturally
morphine, coedeine
Name an opioid full agonist that exists due to synthetic production
dihydromorphine, methadone, pethidine
Name an opioid partial agonist that exists
buprenorphine
Name an opioid antagonist
naloxone
True or false: oxycodone, although a different opioid from morphine, produces similar side effects
True
Why is it important to prescribe laxatives alongside opioids?
The prescribing of laxatives e.g. senna/ lactulose is important as opioid receptors in the gut are targeted and this reduces gut motility leading to constipation. Important to prescribe alongside opioid as it is likely patient will have opioid induced constipation
Why do we prescribe anti-emetics with opioids?
Anti-emetics are prescribed with opioids e.g. dihydramine or ondansterone as the opioids target opioid receptors in the chemo-receptor trigger zone which can lead to nausea and vomitting
What is the main interaction with opioids?
MOA inhibitors used in parkinsons's - hyperpyrexic coma
What are other common interactions with opioids?
sedative hypnotics e.g. antidepressants due to increased risk of respiratory depression
including antipsychotic drugs due to increased sedation
What is arthritis?
Arthritis is an inflammatory/ pain disorder that affects the joints
What are the 3 types of arthritis?
Osteoarthritis
Rheumatoid arthritis
Gout
What are the common symptoms of athritis?
Joint pain, tenderness and swelling
Inflammation surrounding the joints
Restricted movements
Warm skin over the joint
Weakness and muscle wasting
Compare rheumatoid arthritis with osteoarthritis
Rheumatoid arthritis is an autoimmune disease which causes the body's immune system to attack the joints. Whereas, osteoarthritis is a degenerative disease which causes pain in the affected joint/ joints. Rheumatoid arthritis is an autoimmune disease and therefore we see symmetrical inflammation of the joints whereas osteoarthritis is degenerative so swelling and pain are only seen in the affected joints, this may only be one joint. Rheumatoid arthritis can be diagnosed at any age between 30-50 is most common, this is because it is an autoimmune condition so onset can be much earlier. Whereas osteoarthritis is caused by a degenerative disease and diagnosis typically occurs as we age. Therefore, osteoarthritis is more prevalent in older population. Rheumatoid athritis is associated with pain and inflammation of the joints, whereas in osteoathritis, there is no inflammation. Rheumatoid athritis and osteoathritis have different drug regimens. Rheumatoid athritis has a faster onset where osteoarthritis has a much slower onset. Rheumatoid athritis is an autoimmune disease so causes systemic swelling which is not observed in osteoathritis
How does a normal joint work?
Syovium - This contains the synovial fluid, which lubricates the bone
Cartilage - Allows for the bones to move against each other without causing friction
Tendons - The tendons anchor the muscle to the bone
Muscle surrounding the bone
Capsule - surrounds the synovium
How does a joint affected by rheumatoid arthritis work?
In rheumatoid arthritis, the synovium/ synovial fluid is inflamed.
The muscles surrounding the bones are weakened
Inflamed tendons
The capsule is fragmented due to swelling of the synovium
Cartilage - worn down/ thinning
What joints are commonly affected in rheumatoid arthritis?
Most commonly the small bones of the hands and feet but also the wrist, knees, ankles etc
In what gender is rheumatoid arthritis more prevalent?
Women
What are the causes of rheumatoid athritis?
Rheumatoid arthritis is caused by an autoimmune disease. Its cause is not fully known:
Genetic factors may play a role in causing rheumatoid athritis
Can be caused by smoking, eating lots of red meat, and caffeine
Less common in those whose diet is high in vitamin C, and those who drink alcohol only in moderation.
What are the common symptoms in rheumatoid athritis?
Common symptoms in rheumatoid arthritis are:
Inflammation of the joints
Pain in the joints
Heat felt from the skin surrounding the joints
Pain which worsens in the morning
Systemic symptoms such as fever, general malaise/ fatigue, unexplained weight loss
Describe the inflammation seen in RA
In RA, we have symmetrical joint swelling due to autoimmune disease which causes systemic inflammation. Pain tends to be worse in the more morning.
How is RA diagnosed?
RA is diagnosed by the DAS28 score which is the disease activity score and looks at all 28 joints
Erythrocyte sedimentation rate is also used as a measure of inflammation
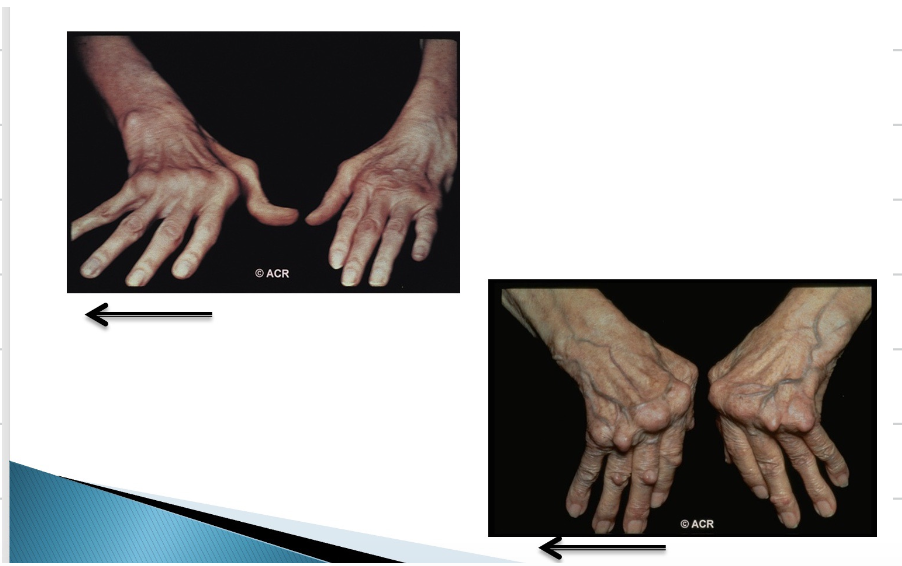

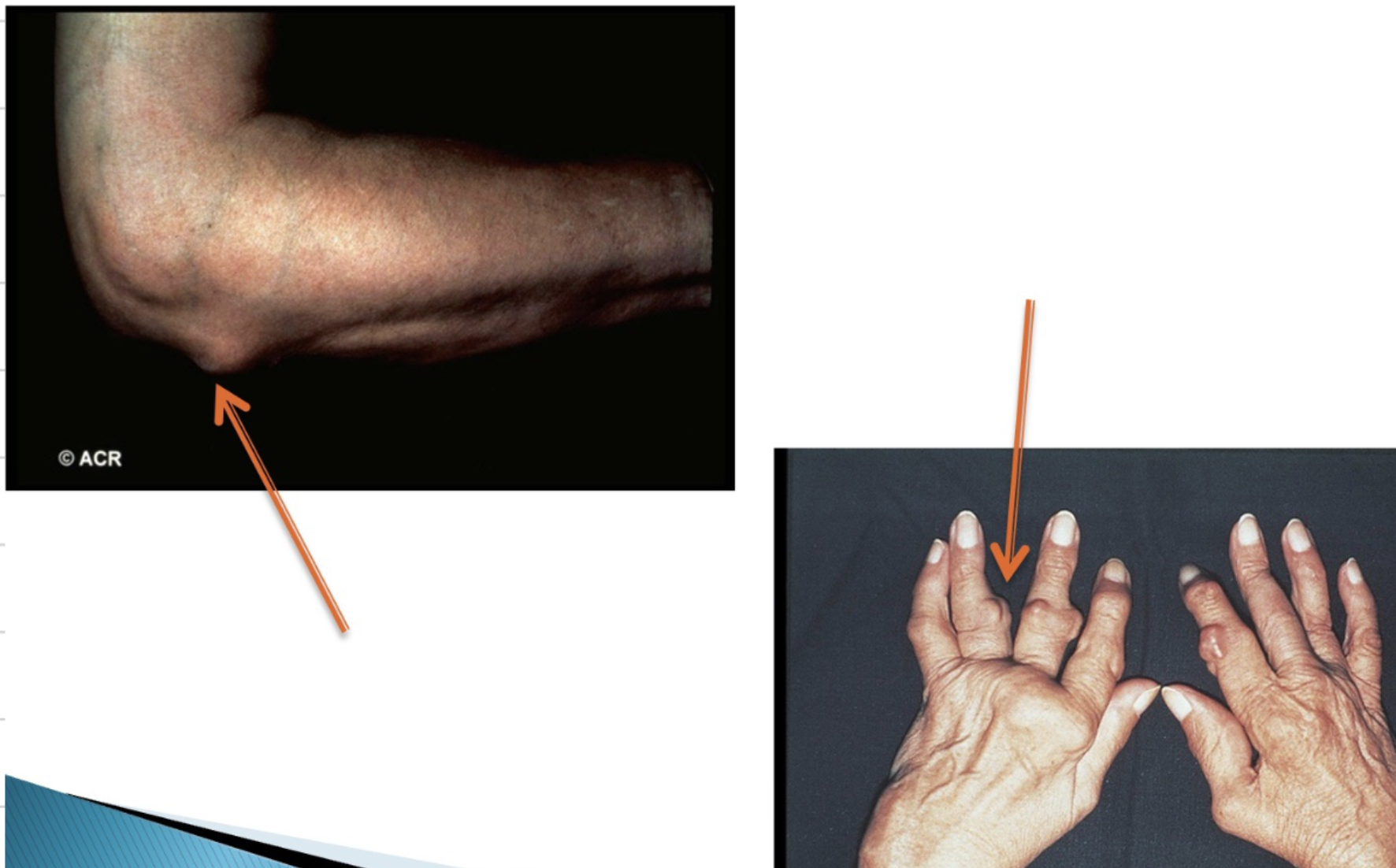
RA causes different deformities of the joint. Explain what is seen in the following image.
Here we can see ulnar deviation whereby there is inflammation of the metacarpophalangeal joints (knuckles) which causes ulnar deviation where the fingers point towards the pinky finger
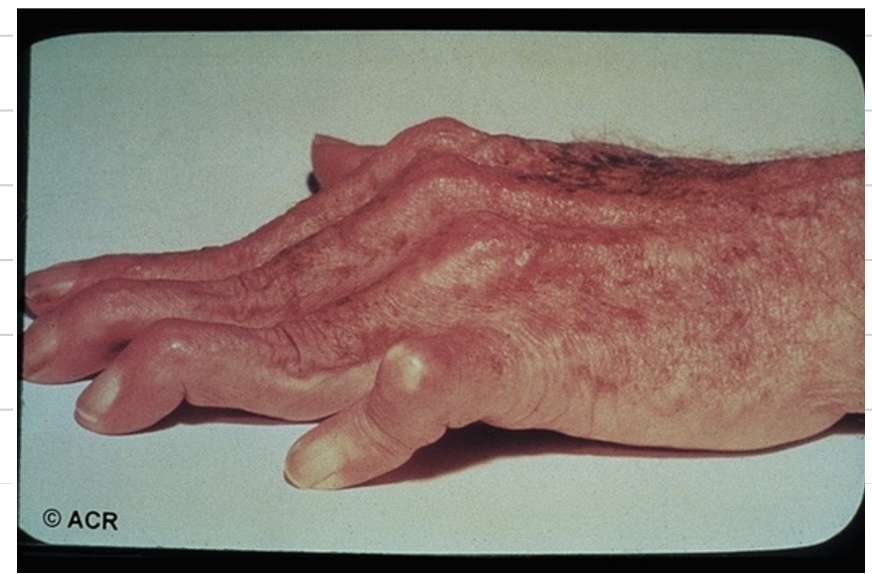
RA causes different deformities of the joint. Explain what is seen in the following image.

Swan neck - This is where we have inflammation of the distal joints and straightening of the middle joints
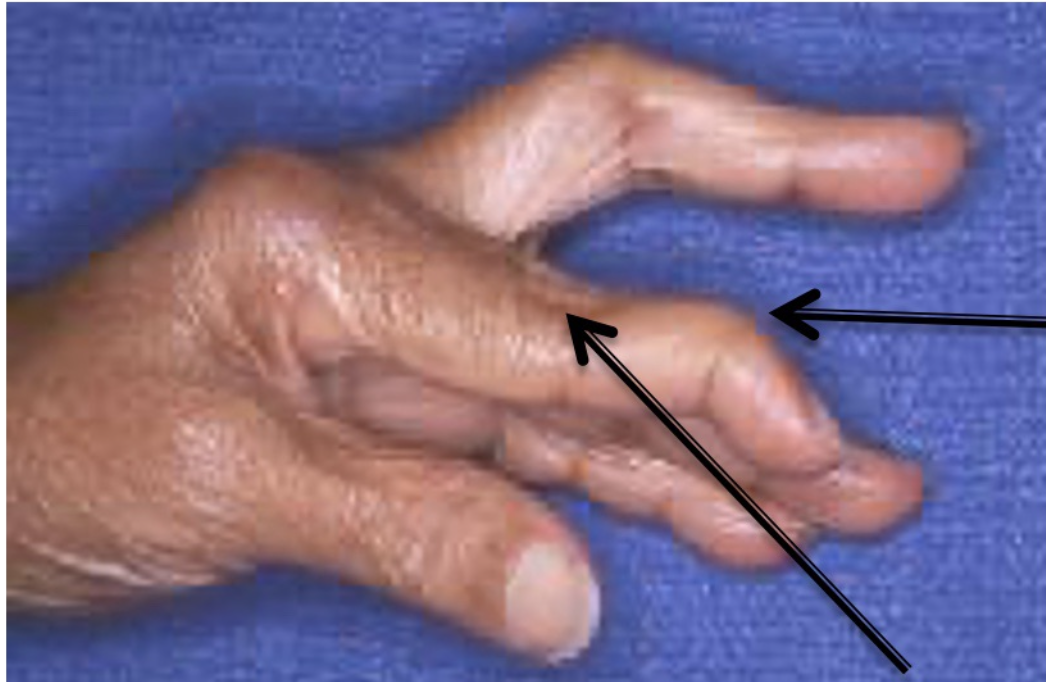

RA causes different deformities of the joint. Explain what is seen in the following image.
Boutonniere’s deformity - This is caused by elevation of the middle joint and deviation in the outermost joint - joint nearest the knuckle is consistently bent towards the palm
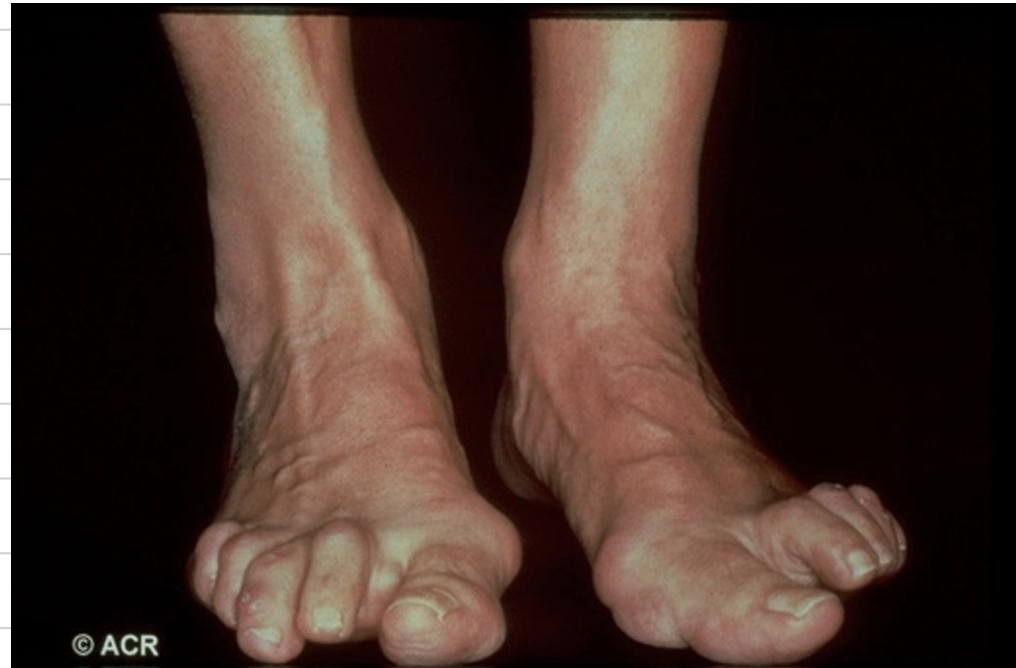

RA causes different deformities of the joint. Explain what is seen in the following image.
Feet deformities are where we see clawing of the toes and formation of bunions on the feet
RA causes different deformities of the joint. Explain what is seen in the following image.
These are rheumatoid nodules and form close to the joints, they are hard nodules under the skin occurring close to the joints
Name examples of systemic involvement in RA
Fatigue and general malasia
Raynauds syndrome
Vasculitis - constriction of blood vessels which can lead to organ damage
Anaemia
Cardiovascular disease
Pulmonary fibrosis
What are the aims of treatment in RA?
In RA we aim to reduce inflammation, reduce pain, preserve joint function, prevent deformity and improve the patients quality of life
True or false: Treatment for RA is lifelong?
True
What drugs are used in the treatment of RA?
NSAIDS - can be both non-selective and selective
DMARDS - disease-modifying anti-rheumatic drugs
Glucocorticoids
Analgesics
What are the two different types of NSAIDs?
Non-selective NSAIDs - ibuprofen, nurofen,
Selective NSAIDs - celecoxib
Note aspirin is non selective but is more selective for COX-1 and hence can be used as a blood thinner
How do non-selective NSAIDS work?
Non-selective NSAIDs are those such as ibuprofen and naproxen. They work by inhibiting the enzyme COX-1 and COX-2 which have a role in inflammation. COX-1 enzyme is involved in reactions which allow for the production of prostaglandins which causes mucus to line the stomach to prevent irritation from stomach acid. It also allows for the production of blood clots. Therefore, when we inhibit COX-1, we inhibit the ability to produce mucus in the stomach to protect it from acid and we have reduced blood clot formation. COX-2 enzyme causes prostaglandins which contribute to inflammation/ activation of inflammatory markers. Therefore, when we inhibit COX-2, we inhibit inflammatory mediator production so reduce inflammation. This is favourable in diseases such as arthritis/ rheumatoid arthritis where we have swelling of the joints.
What are side effects of non-selective NSAIDS?
Non-selective NSAIDs inhibit both COX-1 and COX-2 and as a result…
Reduced inflammation
Increased risk of bleeding due to reduced ability for blood to clot
Stomach irritation as mucus lining of stomach is reduced
Bronchoconstriction
Non-selective NSAIDS are contraindicated in which patients?
Asthmatics - increased risk of bronchoconstriction
AKI - Nephrotoxic drugs should not be used in patients with renal impairment
History of peptic ulcers
How are NSAIDs nephortoxic?
NSAIDs, through inhibition of COX-1, causes reduced blood flow to the kidney. This is because COX-1 is involved in the regulation of blood supply to the kidney. This reduced blood flow to the kidneys can cause AKI and therefore is contra-indicated in renal impaired patients
How do selective NSAIDS work?
Selective NSAIDs such as celecoxib work by inhibiting COX-2 enzyme, they are selective for COX-2 and therefore play a role in reducing inflammation but are not associated with the symptoms seen in non-selective NSAIDs. This means it does not impair mucosal lining of stomach, does not increase risk of bleeding, and does not reduce blood flow to the kidney.
What counselling advice is given with non-selective NSAIDS?
Take with food
Patient may be co-prescribed omeprazole PPI to reduce irritation of the stomach caused by NSAID
What are DMARDs? Name an example of a DMARD
DMARDs are disease-modifying anti-rheumatic drugs. They are able to reduce the inflammation and pain seen in rheumatoid athritis and are first line. The most common and effective is methotrexate. Ability to slow disease progression
Name examples of DMARDs.
Methotrexate
Sulfasalazine
Hydroxychloroquine
True or false: Methotrexate and sulfasalazine can be used in combination
True
Briefly explain what methotrexate is, how it works, onset of action.
Methotrexate is the first-line treatment of rheumatoid arthritis. It is a DMARD - disease disease-modifying anti-rheumatic drug. Methotrexate is a dihhydrofolate reducatase inhibitor. It has a role in inhibiting the production of thymine which is essential for the production of purines and pyridines
Explain the mechanism of action of methotrexate
Methotrexate is a dihydrofolate reductase inhibitor. It inhibits the production of thymine which is required for the production of purines and pyridines and is required for DNA nucleotide synthesis.
Methotrexate works by…
Folate from the diet is converted to dihydrofolate which is converted to tetrahydrofolate. The tetrahydrofolate is then converted to 5,10 methyl tetrahydrofolate which is converted back to dihydrofolate by dihydrofolate reductase. During this process thymidine synathase is activated which is responsible for producing thymine.
Methotrexate is a dihydrofolate reductase inhibitor, and therefore inhibits the activation of thymidylate synthase and the production of thymine. Subsequently, we cannot have DNA nucleotide synthesis in rapidly dividing cells such as immune cells so inflammation reduces.
Why do we give folic acid with methotrexate?
Methotrexate inhibits dihydrofolate reductase enzymes, therefore folate is not being used effectively to produce thymine. We give additional folic acid to allow for the production of folate so that normal cells can continue to synthesis DNA. Methotrexate inhibits the conversion folic acid to folate and therefore causes folate deficiency so more needs to be given to ensure surivial and replication of normally dividing cells.
How do we give folic acid and methotrexate together?
Folic acid can be given once weekly, on a day different to the day you take methotrexate
OR
can be given every day of the week, except the day you take methotrexate
This ensures the folic acid is not affecting the mechanism of action of methotrexate whilst ensuring patients taking methotrexate have sufficient levels of folic acid for normal cell production of thymine
What is a major contra-indication of methotrexate?
Trimethoprim is a major contra-indication with methotrexate - both inhibit folic acid metabolism which may lead to bone marrow suppression
How can biologics be used in the treatment of RA?
Biologics are drugs which come from biological sources such as microorganisms/ animals or humans. They are typically antibodies and are immunosuppressants which suppress the immune system in autoimmune disease.
Explain the different types of biologics used in RA
TNFa inhibitors - TNFa and IL-6 directly lead to the activation of neutrophils which leads to T-cell activation. Therefore, if we inhibit TNFa, then we inhibit neutrophil activation and reduce inflammation. Inhibition of interleukins also depletes the number of neutrophils so reduces inflammation
Interleukin inhibitors - If we inhibit interleukins, we inhibit neutrophil activation and reduce inflammation
T-Cell activation inhibitors - If we inhibit the activation of T-cells then we inhibit inflammation
B-cell depletion - B-cells are antibody-producing cells, if we deplete the number of B-cells then we will deplete antibody production and as a result we deplete auto-antibodies which drive immune response in the autoimmune disease RA where the bodys own antibodies target self-antigens.
What should patients be counselled on when taking biologics?
Biologics cause immunosuppression and as a result patients should be counselled on reducing risks of infection etc due to immunosuppression.
How are glucocorticoids used in RA?
Glucocorticoids are able to reduce inflammation but are used for short-term therapies. DMARDs such as methotrexate and sulfasalazine are first line for RA, however, they have an onset between 4-6 weeks and therefore we use glucocorticoids during this time to provide symptomatic relief of inflammation in RA
What are some side effects of prolonged use of glucocorticoids?
osteoporsis
diabetes
Adrenal gland suppression
Provide a summary drug treatment for an RA patient
DMARDs - methotrexate, and/ or sulfasalazine
Glucocorticoids can be prescribed for short term use (4-6 weeks) whilst waiting for DMARDs to take effect
Folic acid co-prescribed with DMARDs
NSAIDs/ paracetamol can be used for symptomatic reliefe
Occasionally, we use biologics
Review after 6 months to look for possible need to switch medication, monitor side effects, disease progression etc.
What is osteoarthritis?
Osteoarthritis is a degenerative disease which affects the joints in an asymmetrical manner. It prevalence increases with age. The onset of the disease is typically slow and inflammation is not seen in the joints. Osteoarthritis causes pain which worsens when pressure is applied to the joints. Patients who are overweight are more likely to suffer from osteoarthritis due to increased pressure on the bones.
Describe what is observed in a joint with osteoarthritis.
In a joint with osteoporosis, we do not see inflammation of the synovium/synovial fluid
The muscle surrounding the bone is weakened
The bone is usually thickened and deformed due to osteophyte activity
Cartilage is worne down
The capsule surrounding the synovium is tightened
Explain the symptoms of OA
pain in the joint which worsens when moving
Stiffness of the joints which worsens with rest
Crunching of the joints during movement
Swelling of the joints - not as common but may occur due to osteophyte activity
Not being able to move the joint as freely as normal
What does disease progression look like in OA?
Joint deformities
Joint pain
Daily movement/ activity is impaired e.g. walking, climbing up the stairs etc
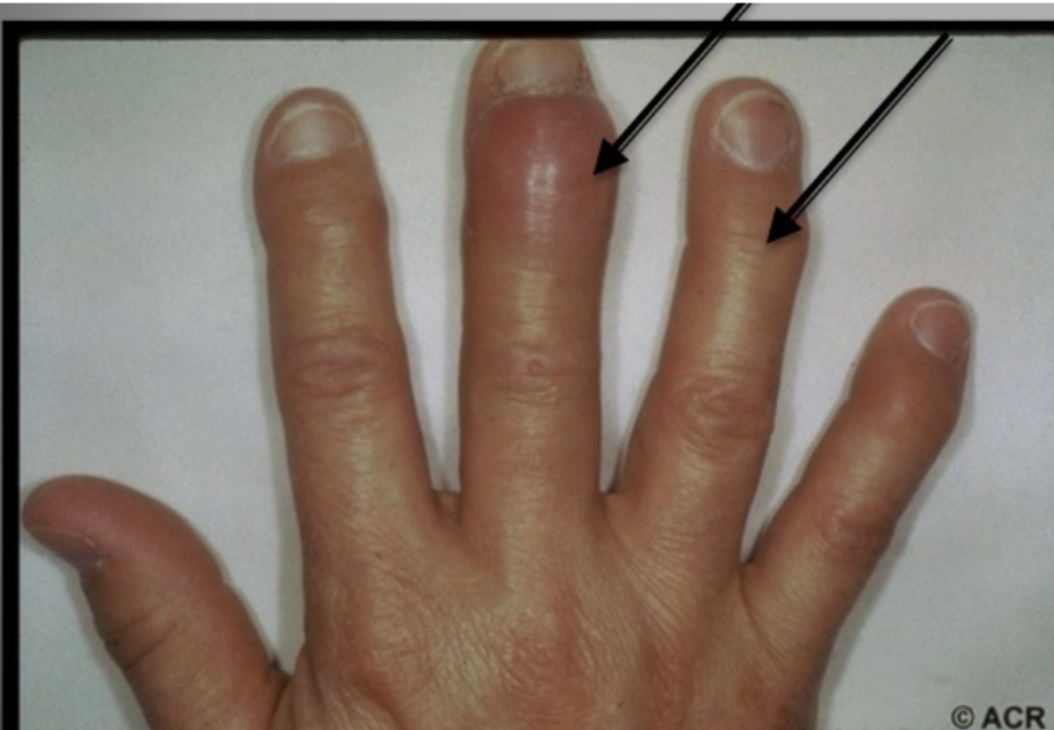
Explain what is seen in the following image

Osteophyte acitivity which causes swelling near the joints - osteophytes are bony lumps which grow on the bone.
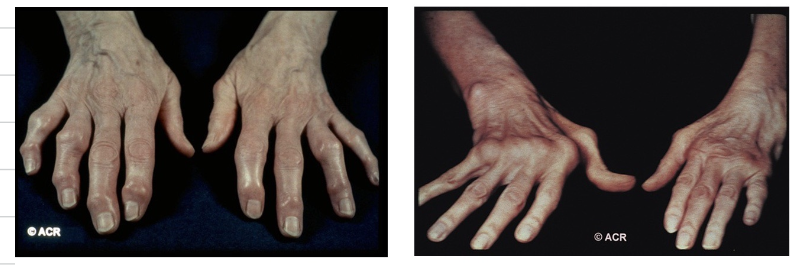
From the following image, explain which photo resembles OA and RA
On the left we can see OA, identified by osteophyte activity (hard lumps on the bone). RA is identified by ulnar deviation which is symmetrical in nature due to autoimmune disease
Explain the symptoms of OA/ what a patient presenting with OA would may experience
Pain in joint which worsen on activity
Difficulty performing day-to-day activities
Joint warmth and/ or tenderness
Muscle wasting and weakness
Bone swelling may occur - osteophyte activity causing hard lumps to form on the bone
What may we expect when conducting a medical history in OA?
Possible known trauma to the joint which may have caused OA
Obesity
No systemic symptoms
Onset should be slow
Patients tend to be older
functional impairment - how is it affecting day-to-day life
How is OA managed?
Analgesics - e.g. paracetamol is used regularly and not PRN - 4g daily
NSAIDs - Can be topical NSAIDs or oral NSAIDs first line is ibuprofen but side effects should be monitored
Local corticosteroid injections can help with inflammation upon flare ups
Physiotherapy
Surgery
Weight loss if the patient is overweight
True or false: Self-management is very important in the treatment of OA?
True
What self-management would we recommend to a patient with OA?
We can recommend:
Loosing weight if the patient is overweight as this reduces the pressure on the joints
Strength-training exercises which can help to strengthen the muscle surrounding the bones
Aerobic exercises - can aid in cardio-fitness and weight loss
Physiotherapy
What does physiotherapy aim to achieve in OA?
Physiotherapy helps to prevent disease progression and preserve the function of the joint. It can help to strengthen the muscle surrounding the joints to reduce pain.
When would we recommend surgery in OA?
Surgery is recommended when daily activities are affected by the deterioration of the joints. Surgery is also recommended when pain is excruciating and not managed with analgesics. Knee replacements are becoming increasing common among OA patients.
What is first-line drug therapy of OA?
Paracetamol 1-2 4 times a day. This is regular use and not PRN
What are additional drug treatments for OA?
Additional drug treatments for OA include:
NSAIDs (not first line) - This can be topical or oral delivery. Ibuprofen is first line but side effects should be monitored and long-term use is not recommended
Other topical treatments include: Capsaicin cream which is derived from chillis and causes pain signals to worsen before they improve. Patients should be counselled on this
Local corticosteroid injections can be used to control inflammation in flare-ups
Why is weight reduction recommended in OA?
Typically, patients with OA present with obesity which increases pressure on the joints, accelerating disease progression. Loosing weight is important to reduce the pressure and strain on the joint caused by excess fat whilst also prioritising strength-training exercises to strengthen the muscles surrounding affected joints.
What is gout?
Gout is a type of inflammatory arthritis. Gout causes inflammation and pain of the joints in people with hyperuricaemia
Explain what causes gout
Gout is caused by hyperuricaemia whereby uric acid levels in the blood are too high which leads to the formation of urate crystals in the joints. Uric acid is produced from purine production. 2/3 of purine production comes from our body cells and 1/3 of purine production comes from the food/ drink we consume. When purines are too high and uric acid levels increase, it increases the risk of urate crystals forming and being deposited in the bone. This accumulation of urate crystals can occur for sometime before symptoms arrive and this is because it is the shedding of the crystals into the synovium fluid which drives inflammation.
What are the risk factors of gout?
Risk factors of gout include:
Reduced excretion of uric acid from the kidneys e.g. in impaired kidney function. This means more uric acid is being reabsorbed into the blood and can lead to urate crystal formation.
Medications such as diuretics
Foods rich in purines such as red meat, sea food, and yeast.
Excessive alcohol consumption which includes beer, stout etc which is derived from yeast
Conditions such as psoriasis
Genetic predispistion
obesity
Hypertension
What is joint is commonly affected in gout?
The hands, elbows, knees, wrists etc can all be affected but most commonly is the joint at the base of the big toe.
Explain what the joint would appear like in a patient with gout.
A joint with gout would have red, shiny skin and appear inflamed and hot to the touch. It would also be very painful.
How can we diagnose gout?
Diagnosis of gout can include:
A blood test to look at levels of uric acid in the blood to test for hyperuricaemia
An X-ray which looks at the formation of urate crystal deposition in the bone
An extraction of the synovium fluid, which looks for the presence of urate crystals in the synovium fluid.
How do we treat gout?
ICE - Ice, compression, elevation and REST
NSAIDS - typically ibuprofen to reduce inflammation. Short term use only 7-14 days
Corticosteroids may be used for inflammation during flare ups
Colchicine
Allopurinol/ febuxostat
List the long-term treatments used in gout.
Colchicine -symptomatic relief/ not uric acid lowering
Allopurinol - uric acid lowering
Febuoxostat - uric acid lowering
Explain how colchicine is used in the treatment of gout.
Colchicine is used to reduce the inflammatory response to urate crystals being deposited in the joint. This provides symptomatic relief of gout but has not effect on the reduction of uric acid levels in the blood.
How is allopurinol used in the treatment of gout? Explain its mechanism of action also.
Allopurinol is a uric acid lowering agent which reduced levels of uric acid in the blood. Allopurinol is first line and should be used as long-term therapy for patients with gout. Allopurinol is a xanthine oxidase inhibitor. Xanthine oxidase is an enzyme which converts xanthine to uric acid. Therefore, if we inhibit xanthine oxidase, we inhibit the conversion of xanthine to uric acid. This therefore helps to reduce uric acid levels in the body and so will prevent the risk of forming urate crystals.
If allopurinol is not tolerated, what uric acid-lowering agent is used instead?
We can instead use febuxostat which is a uric acid lowering agent with a faster onset than allopurinol. it is a more selective xanthine oxidase inhibitor
Allopurinol/ febuxostat may take a while to take effect, what can be prescribed in the meantime?
Colchicine can be prescribed whilst waiting for allopurinol/ febuxostat to take effect because it provides symptomatic relief of the inflammation by reducing the inflammatory response to urate acid. Can be used for up to 6 months following initiation of allopurinol/ febuoxostat
How do diuretics cause gout?
Diuretics can cause gout because they increase the reabsorption of uric acid from the kidney. Diuretics increase sodium and water excretion which in turn may increase uric acid reabsorption. This can cause uric acid levels to increase, increasing the risk or urate crystal formation, causing gout.
How does psoriasis increase the risk of gout?
Psoriasis causes an increased cell turnover rate by increasing the production of cells we increase purine synthesis. Subsequently, we increase uric acid levels in the body and allow for the formation of urate crystals in the joint.