Chem 11E Unit 7 - Molecular Polarity and Solubility
1/32
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
what are the van Der Waals forces?
London forces
dipole-dipole forces
hydrogen bonds (stronger version of dipole-dipole)
what’s the distinction between the 2 types of forces due to?
the presence or absence of permanent dipoles
what’s a dipole?
a partial separation of charge existing when one end of molecule or bond has slight excess of positive charge and the other end has slight excess of negative charge
what are London forces?
weak attractive forces existing from temporary dipolar attraction between neighbouring atoms
what are dipole-dipole forces?
bonding forces from an electrostatic attraction between molecules having permanent dipoles
if a permanent dipole is absent what forces are present? when is it present? if a permanent dipole is present?
only London forces
London forces is ALWAYS present (even when permanent dipoles or ionic)
dipole-dipole + London forces
what happens when 2 atoms bond?
since most atoms bonding have different electronegativites, this gives rise to a dipole (ex: H - Cl)
𝛿+ and 𝛿–?
slight excess in positive charge
slight excess in negative charge
what happens to the atoms with low electronegativites?
tend to form positive ions, are electropositive (alkali metals, alkali earth metals)
what’s molecular polarity?
a molecule is polar if the distribution of charge within the molecule is uneven
what affects molecule polarity (5 things)?
electronegativity of the atoms
polarity of the individual bonds within the molecule
intermolecular forces
shape of the molecule
symmetrical shape or not
if a molecule has polar bonds that are arranged symmetrically what is it?
non polar, because bonds must be polar and angles not equal to be polar molecule as symmetrical = forces resolved, charge is evenly distributed throughout molecule (rock and ropes example)
what are dipole-dipole forces weak equivalents of? why?
ionic bonds, because both are forms of attraction between a more positively charged atom or molecule and a more negative atom or molecule
relative strengths of interactions?
ionic bond » dipole-dipole forces ≈ London forces
what are hydrogen bonds?
a relatively strong type of dipole-dipole attraction occurring when H is bonded with N, O, or F (due to large difference in electronegativities)
when will a hydrogen bond form when bonded with N, O, or F?
HF ONLY
NH, NH2, NH3
OH, H2O, H2O2
what happens to viscosity when molecules have stronger intermolecular bonds vs. weaker? why?
stronger intermolecular bonding = higher viscosity (more resistant to flow) due to higher melting points and lessened ability for molecules to “slide“ and “flow“
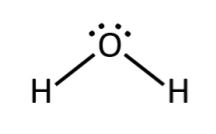
polar or nonpolar - H2O?
bent
charge not evenly distributed so polar
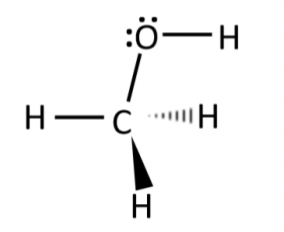
methanol (CH3OH)
polar
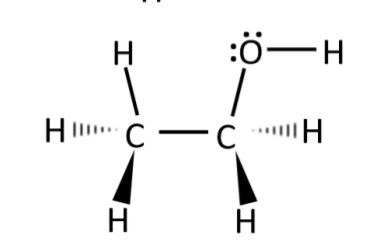
ethanol (CH3CH2OH)
combo of tetrahedral and bent
hydrogen bonding
polar
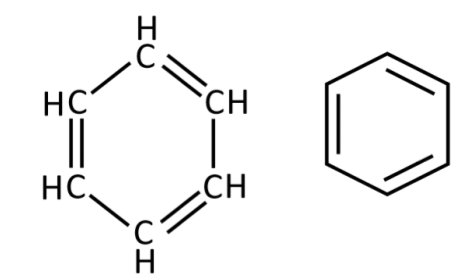
Benzene (C6H6)
non-polar
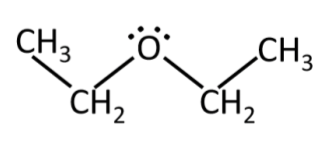
ethoxyethane aka diethyl ether, ether, hospital ether
polar
(C2H5)2O
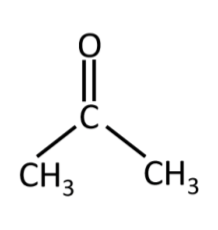
acetone (CH3COCH3)
nail polish remover
polar
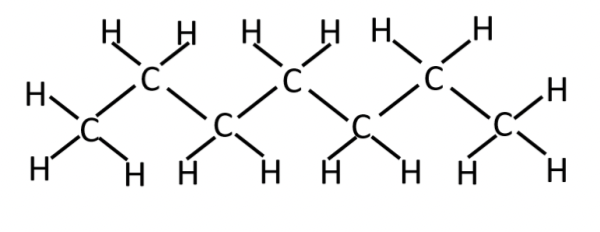
heptane (C7H16)
non-polar
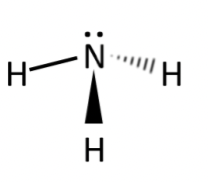
ammonia (NH3)
polar
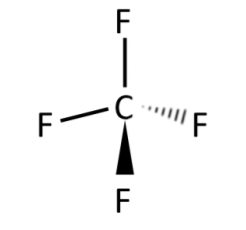
CF4
tetrahedral
all angles 109.5 degrees
bonds evenly distributed so non-polar
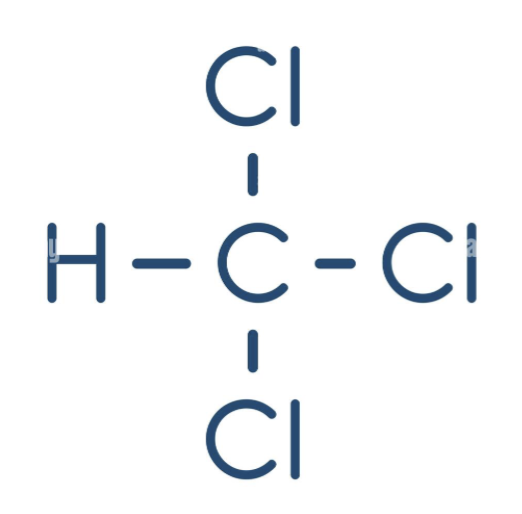
chloroform (CHCl3)
polar
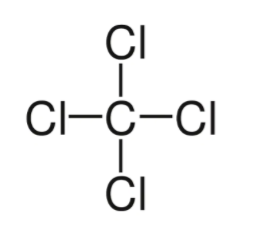
carbon tetrachloride (CCl4)
non-polar
dissolving process in 3 attractions?
explanation
why does polar dissolve polar? why does non-polar dissolve non-polar?
explanation
water, methanol, and ethanol dissolving abilities?
explanation
polar dissolving polar 2 examples + 1 non example?
sugar dissolves in water
NaCl dissolves in water
oil does not dissolve in water
non-polar dissolving non-polar 1 example + 1 non example?
iodine dissolves in oil
iodine does not dissolve in water