rate of reaction
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
collision theory - for the particles to react, they must do three things
- collide
- must have the minimum energy required to react (activation energy)
- collide with the correct orientation
Factor 1 that affects the rate of reaction
concentration - increased concentration means more particles in the same space so more collisions. rate of reaction increases. double concentration = double collisio
factor 2 that affects rate of reaction
pressure - the same number of particles, but we reduce the volume of the container rather than increase the number of particles
factor 3 that affects rate of react
temperature - increase temp = increase rate of reaction. Particles have more kinetic energy, so they collide more frequently (with more energy). More particles have at least the minimum activation energy required to react. increased frequency of successful collisions
factor 4 that affects the rate of reaction
surface area - increasing surface area provides particles with more sites or surfaces for collision so more space for collisions to occur. rate increases. increase number of collisions. increase frequency of successful collisions
exam question - explain using collision theory what happens to the rate of reaction when the volume of the container the particles are in is increased?
4 mark answer:
- Increasing volume will decrease pressure
- This causes particles to collide less frequently as they are further apart
- therefore, the less frequent successful collisions
- rate of reaction decreases
catalyst
a chemical that helps speed up a reaction by offering an alternative reaction pathway with lower activation energy
exam question - explain how catalysts affect the rate of reaction using collision theory
4 mark answer:
- Catalysts offer an alternative reaction pathway with a lower activation energy
- more particles have the minimum activation energy required
- increase frequent successful collisions
- rate of reaction increases
How to calculate the mean rate of reaction from a graph
- quantity of product formed / time taken
or
- quantity of reactant used / time taken
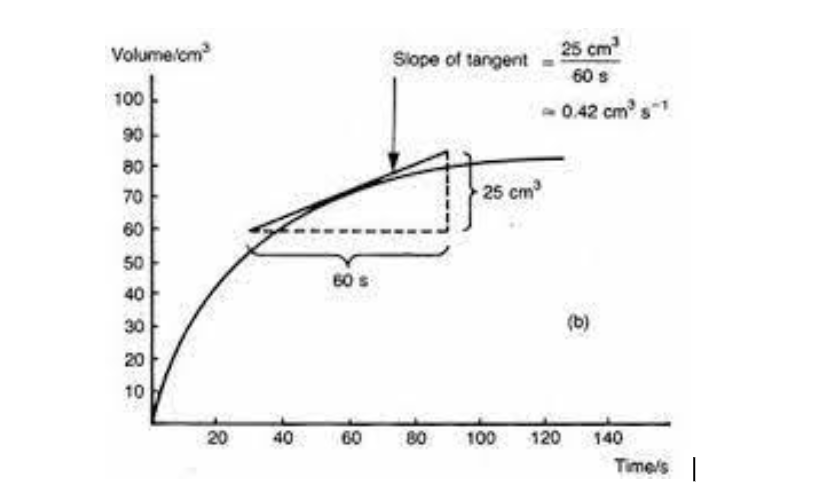
how to find the rate of reaction from a tangent
explanation

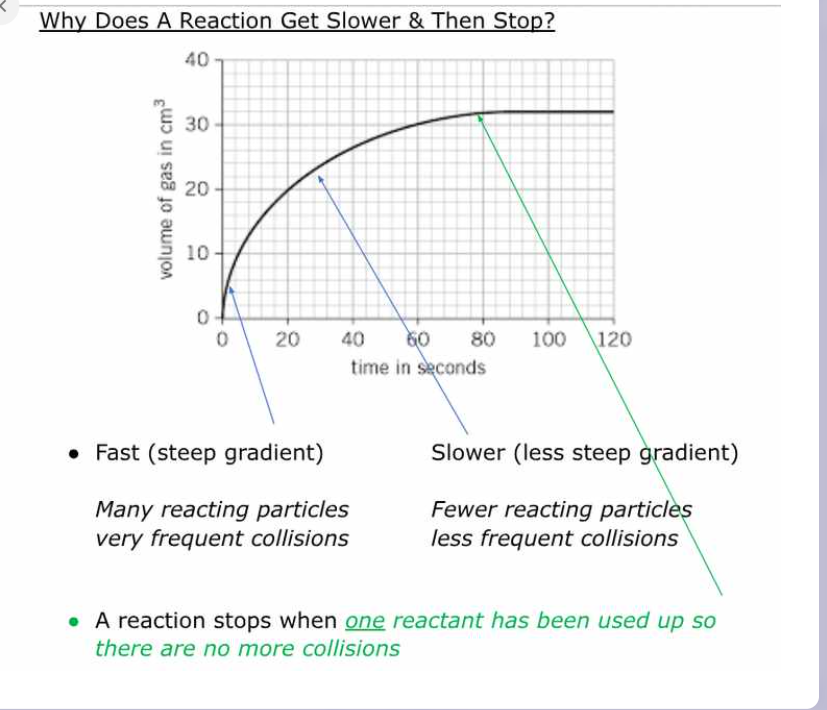
photo explanantion
from book reacton progress graph -
initial rate is fast with lots of reacting particles colliding
rate slows. Successful collisions occur less frequently
rate is 0. One or more reactants have been used up
- the steeper the line on the graph, the faster the rate of reaction
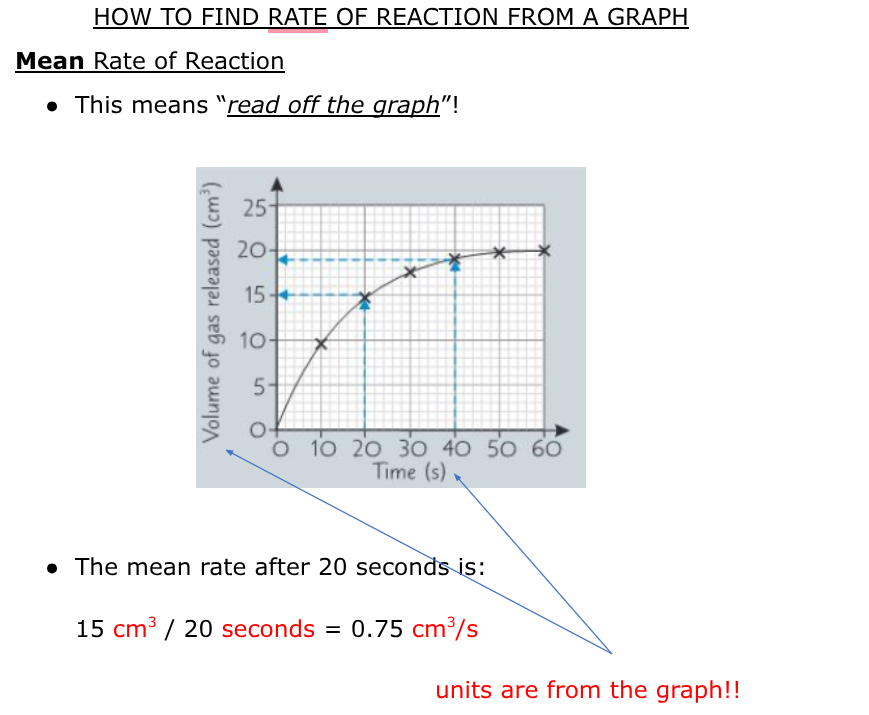
how to find mean rate of reaction
mean rate between two times formulas


required practical - measuring the increasing volume of gas given off.
If a reaction produces a gas, you can use the gas to find out the rate of reaction. You do this by collecting the gas and measuring the volume given off at time intervals.
- saftey: wear eye protection
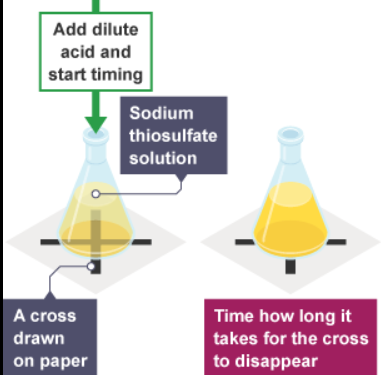
required practical - measuring the decreasing light passing through solution
Some reactions in solution make a suspension of an insoluble solid (precipitate). This makes the solution go cloudy. You can use this to measure the rate at which the precipitate appears.
- safety: toxic gas is produced from the reaction, so don't inhale in the flask