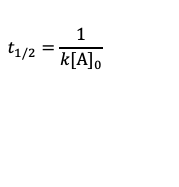FUNCHEM 18: Rate Laws and Order of Reactions
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 3:12 PM on 12/21/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
How can we define the reaction order
Initial Rates
Graphical Method (Integrated Rate Law)
Half Life
2
New cards
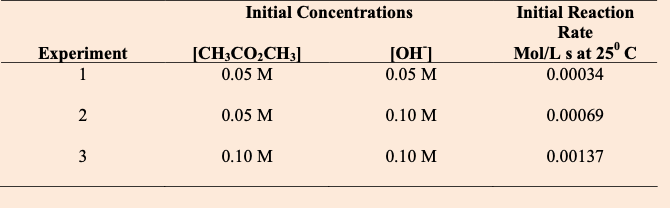
Find the rate equation
CH3CO2CH3 + OH- è CH3CO2- + CH3OH
Rate= 0.136(CH3CO2CH3) (OH- )
3
New cards
What’s the formula for zero order reaction in graphical methods

4
New cards
What’s the formula for first order reaction in graphical method
ln [N2O]t = -kt + ln [N2O]o
5
New cards
What’s the formula for the second order reaction in graphical method
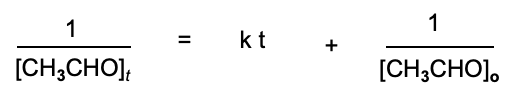
6
New cards
What’s the formula for the half life of zero order reaction
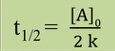
7
New cards
What’s the half life of first order reaction
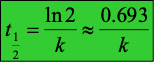
8
New cards
What’s the half life of second order reaction