Chemistry Formulas
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Standard Temperature and Pressure

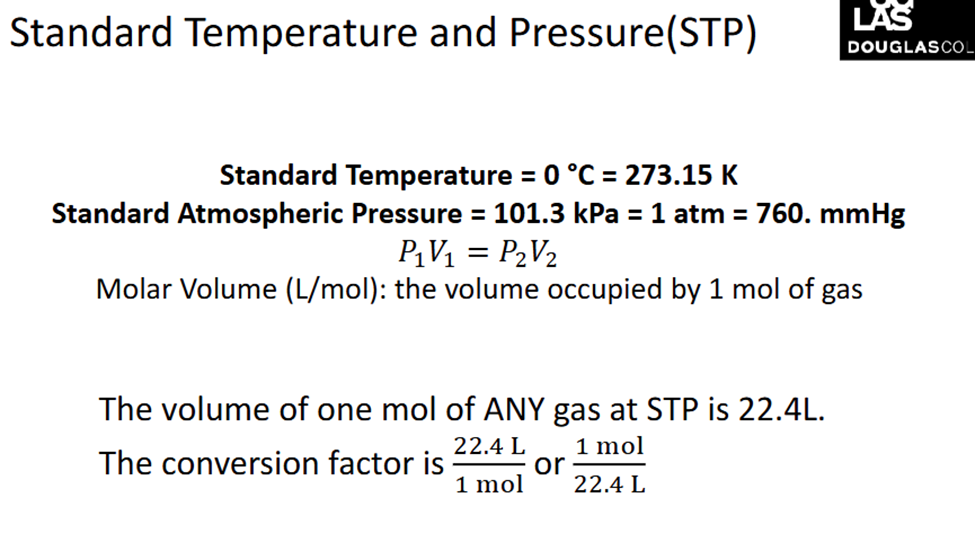
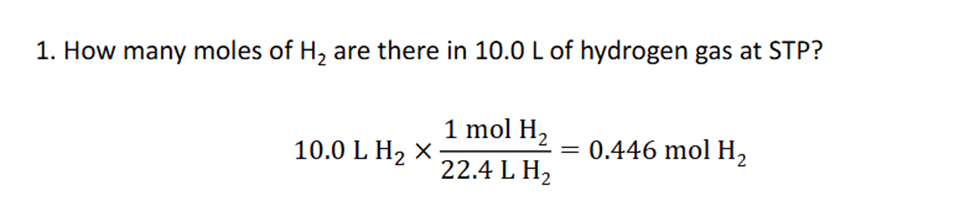
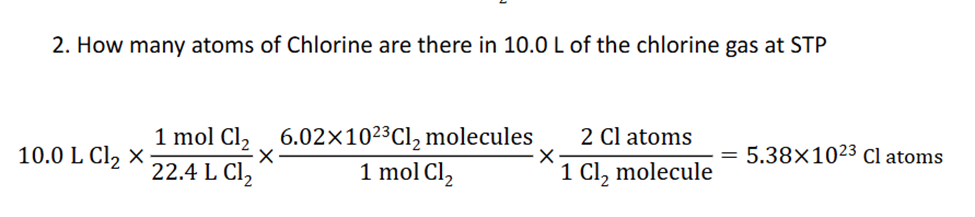
Ideal Gas law
The ideal gas law is a mathematical relationship between pressure, volume, temperature, and the number of moles of a gas. (Tempereature has to always be in kelvin)


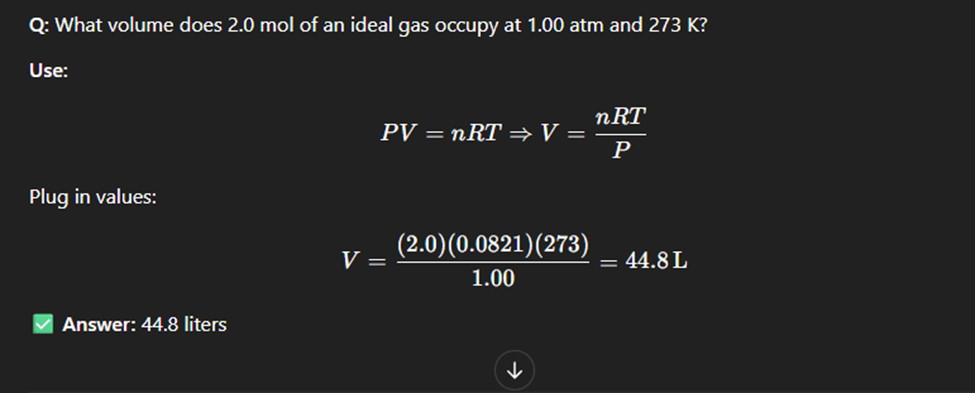
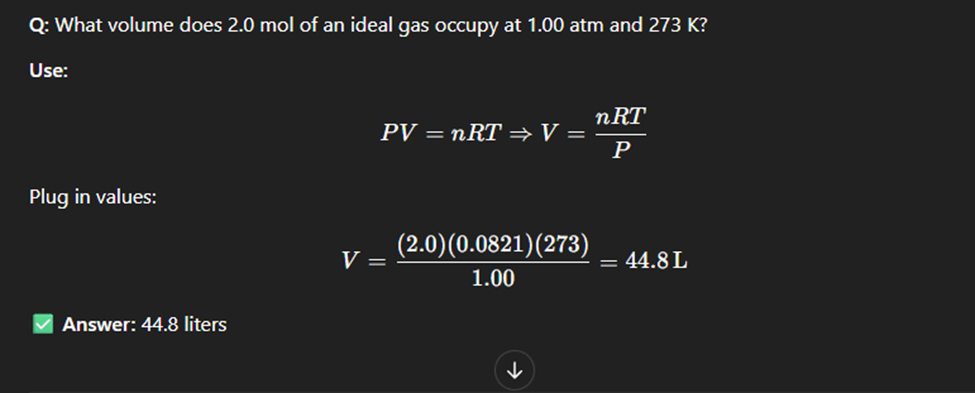
Avargado law
At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas.
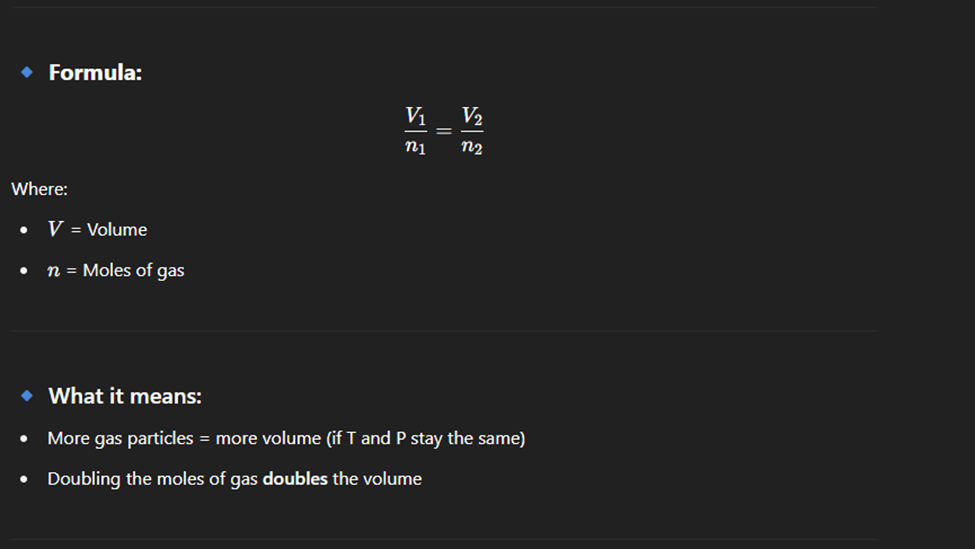
Charles law
At constant pressure, the volume of a gas is directly proportional to its temperature in Kelvin.
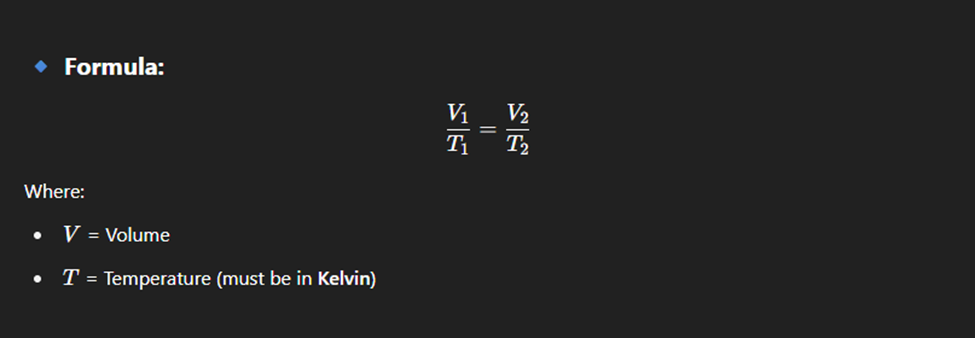
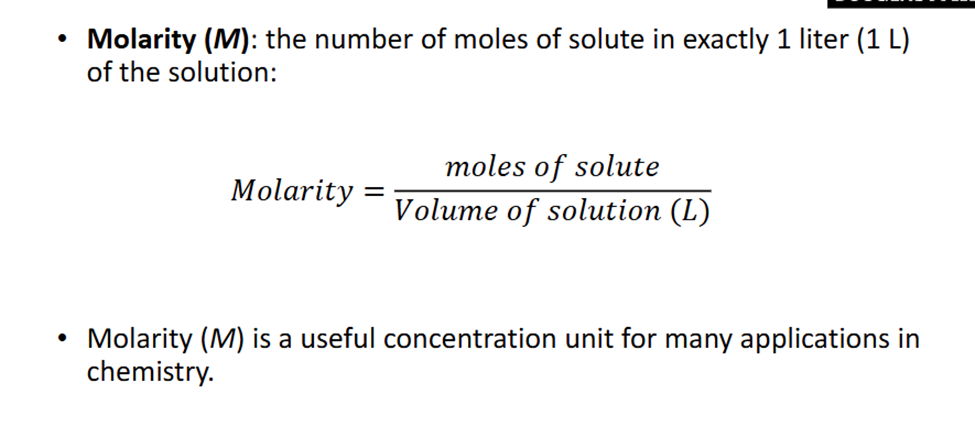
Molarity
Number of Moles in excatly 1L of the solutin
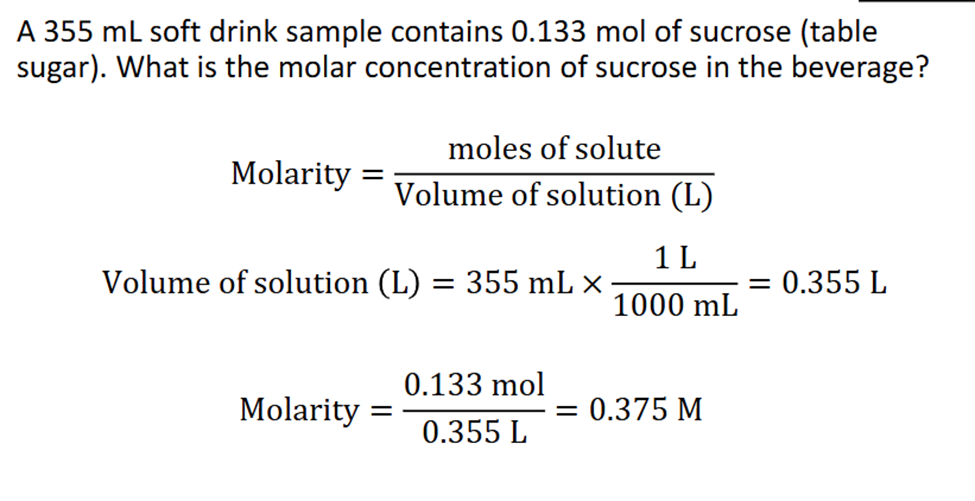
Moles and Concentration

Concentration (M)
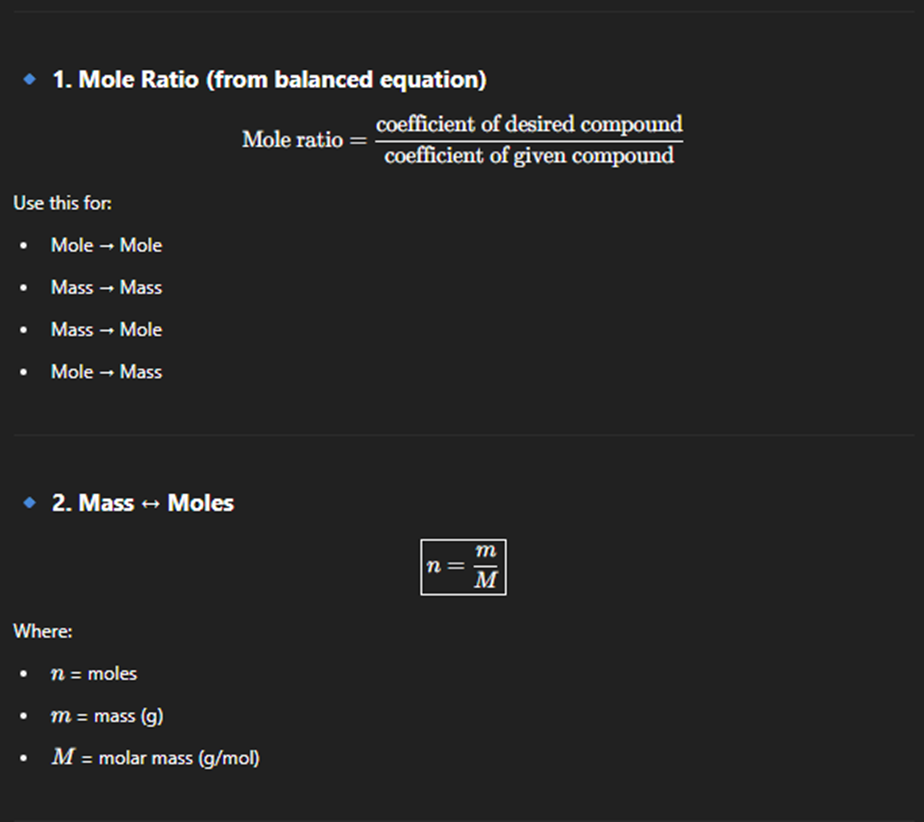
Rate reaction
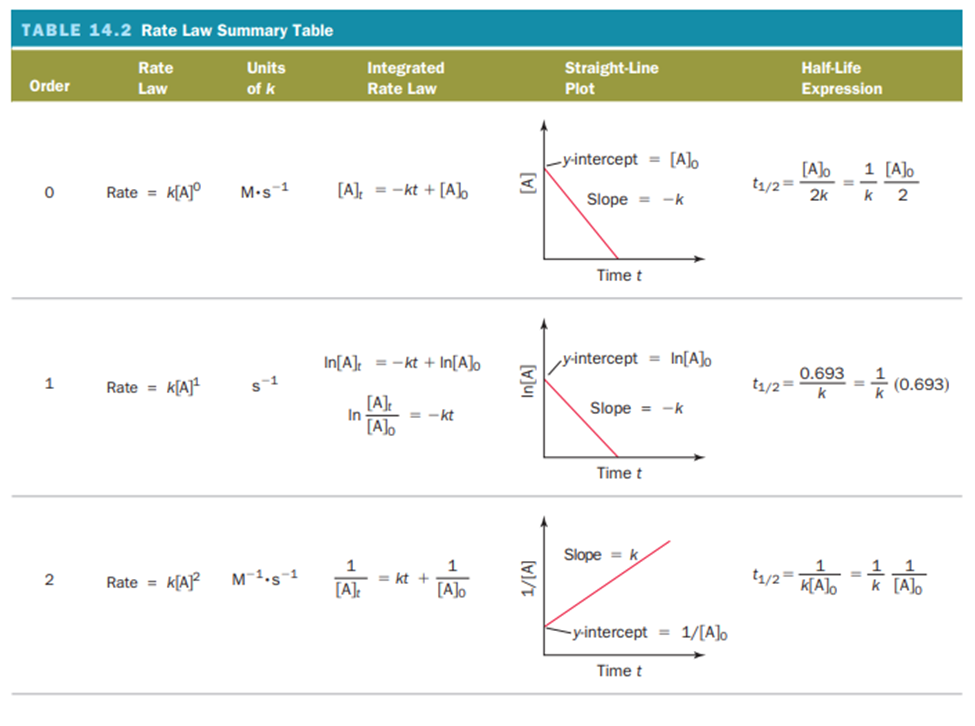

Le Châtelier’s Principle
Definition:
When a chemical system at equilibrium is disturbed, the system shifts in a direction that minimizes the disturbance.
What It Means:
Equilibrium acts like a balance — when disturbed, the system "pushes back" to restore balance.
This response helps the system re-establish equilibrium.