Reactions of acids: Chemical changes: Chemistry: GCSE (9:1)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Metal + Acid -->
Salt + hydrogen
Acid + Base -->
Salt + water
Acid + Carbonate -->
Salt + water + carbon dioxide
Hydrochloric acid
HCl
Sulfuric acid
H2SO4
Nitric acid
HNO3
Type of salt produced by hydrochloric acid
Metal chloride
Type of salt produced by sulfuric acid
Metal sulfate
Type of salt produced by nitric acid
Metal nitrate
Acid
A substance that increases the H+ ion concentration of a solution
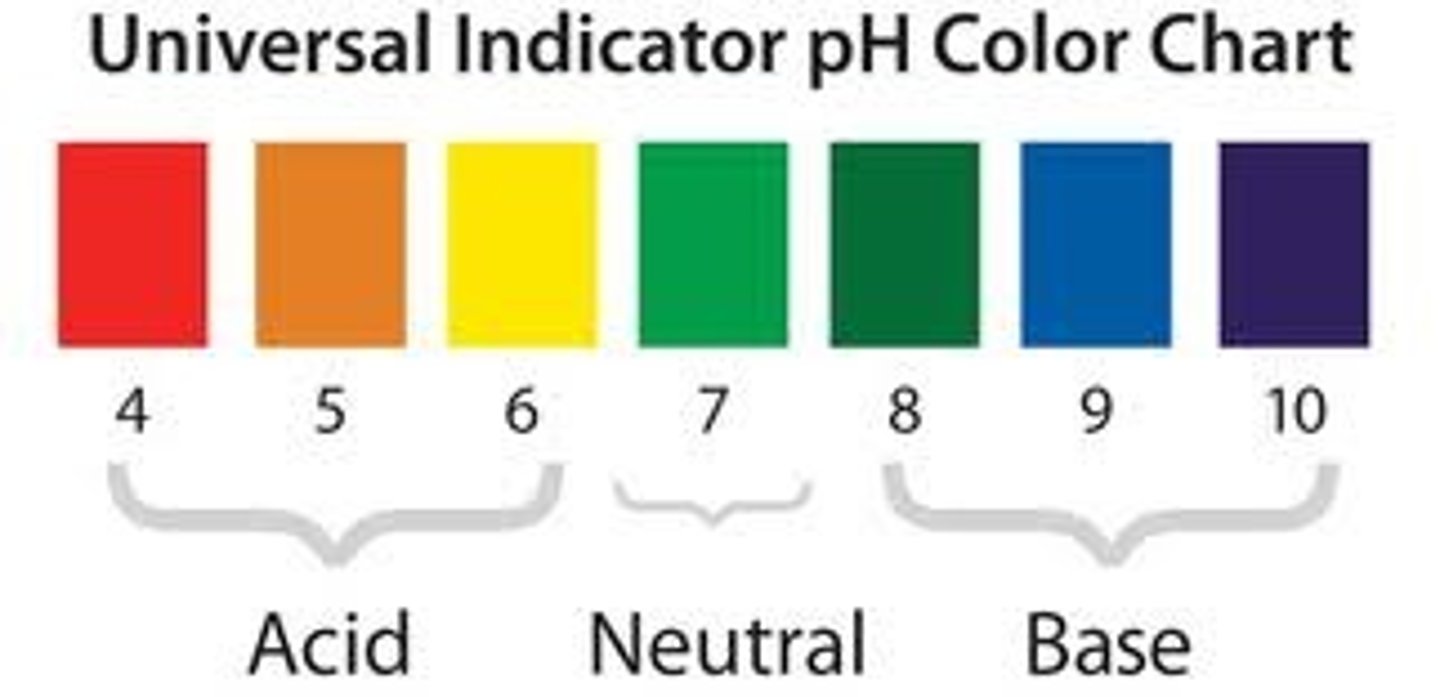
Base
A substance that reacts with an acid and neutralises it
Alkali
A soluble base, that produces OH- ions in solution
Types of chemicals that are bases
Metal oxides, metal hydroxides, metal carbonates, ammonia
Types of chemicals that are alkalis
Metal hydroxides, ammonia
Neutralisation reaction
The reaction of an acid and a base forming a salt and water
Ionic equation for neutralisation
H+ + OH- --> H2O
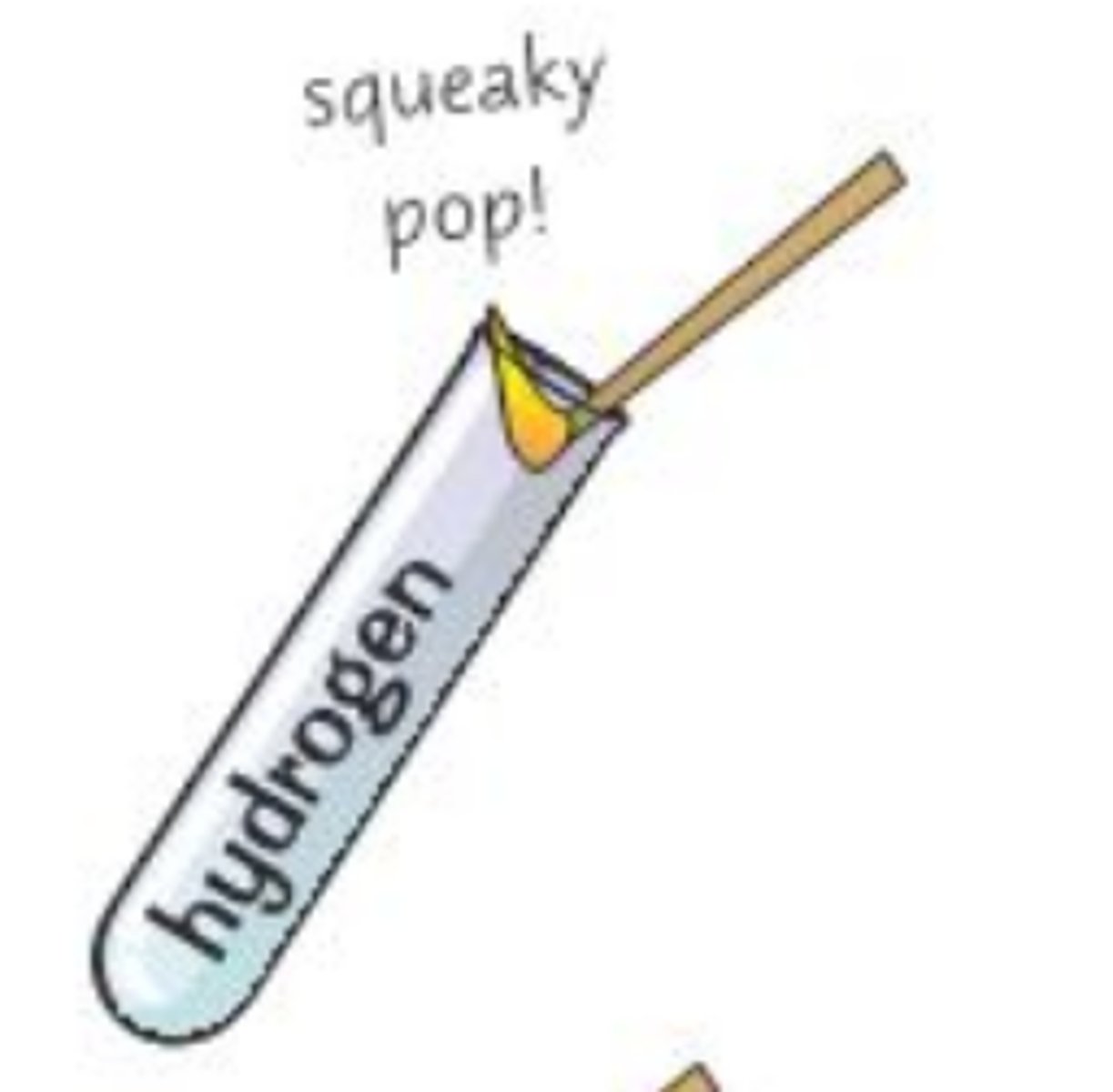
Test for hydrogen gas
Lighted splint burns with a squeaky pop.
Test for carbon dioxide gas
Bubble through to limewater forming a white precipitate
