ORGO 262 Exam 1
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Classify the following substituents according to whether they are electron donors or electron acceptors relative to hydrogen by the resonance and the inductive mechanisms.
INDUCTIVE EFFECT:
If the atom at the point of attachment to the benzene has a:
higher electronegativity than H, then it's an acceptor by the inductive effect
lower electronegativity than H, then it's a donor by the inductive effect
RESONANCE EFFECT:
If the atom at the point of attachment to the benzene:
is electron-rich (has LPE's, or is negatively charged) then it is a donor by the resonance effect
is electron-poor (has no LPE's, or is positively charged) then it is an acceptor by the resonance effect.
Draw the least stable resonance form for the intermediate in the following electrophilic substitution reaction.
Include all lone (unshared) electron pairs and formal charges.
Electron acceptors destabilize resonance forms owing to accumulation of positive charge at the ring carbon bearing the substituent.
Resonance electron donor groups stabilize resonance forms by delocalizing adjacent positive charge while forming a pi bond to the ring atom.
This is the basis for the effect of substituents on orientation and reactivity in electrophilic substitution reactions.
In both series below the three aromatic compounds illustrated undergo the electrophilic substitution reaction shown
Reaction: Nitration
Which compound (A, B, or C) reacts the fastest?
Which compound (A, B or C) reacts the slowest?
The relative rates of reaction of the aromatic compounds shown in electrophilic aromatic substitution reactions reflect the activating or deactivating effects of the substituents on the benzene ring.
Activating -> faster reaction than benzene
Deactivating -> slower reaction than benzene
Indicate, by letter(s), the position(s) on the ring at which substitution occurs when the aromatic compounds shown undergo bromination with Br2, FeBr3 (when necessary).
Look at the chart given to you for the exam
Draw structural formulas for the major organic product of the reagents shown. (Tri-substituted aromatic ring)
A + A : the stronger activator will determine the position of the substituent
A + D: the activator will determine the position of the substituent
D + D: the weaker deactivator will determine the position of the substituent
Select the reagents you would use to synthesize the compound below from benzene. More than one step is required. If no third step is needed, leave that entry blank.
(red = O, blue = N, ivory = H, green = Cl, red-brown = Br, yellow = S)
A. Br2, FeBr3
B. Cl2, FeCl3
C. HNO3, AlCl3
D. CH3Cl, AlCl3
Zn,Hg/ HCl
E. Ch3COCl, AlCl3
F. KMnO4, H2O
G. H2, Pd/C
H. SO3, H2SO4
I. NaOH, H2O
J. N-bromosuccinimide (NBS)
K. 1) H2NO3, H2SO4
2) Fe, H3O+
L. 1) Fe, H3O+
2) OH-
M.OsO4/ NaHSO3
N. O3/ Zn
O. KOH
P. BH3/ H2O2
KNOW what these reagents are used for!
a. Substitutes a hydrogen for a Br
b. Substitutes a hydrogen for a Cl
c. Substitutes a hydrogen for an NO2
d. Substitutes a hydrogen for the carbon chain attached to a halide.
Zn Hg treated with HCl can get rid of any oxygens on the new substituted group.
e. substitutes a hydrogen for OCCH3.
F. oxidation: will replace BENZYLIC hydrogens with COOH
g. will get rid of ALL oxygens on the molecule
h. sulfuration - substitutes a hydrogen for an SO2
i. acid-base reaction
j. bromination
k. 1) attaches an NO2
2) reduces the NO2 group to an NH2
l. only reduces the nitro group to NH2
m. adds OH's to both sides of each pi bond
n. splices molecule into C=O fragments
o. base; elimination of H and halide (ex.Br) from and forms a double bond
p. antimark. addition of OH and H
Consider which reaction should go first. This will determine whether the second substituent has ortho/para/meta orientation.
Identify each of the following groups as an activator or deactivator and as an o,p-director or m-director.
Feedback:
1st group: The pair of electrons on the atom directly attached to the ring stabilizes a positive charge on the attached benzene ring. The effect is greatest at the ortho and para positions.
2nd group: The pair of electrons on the atom directly attached to the ring stabilizes a positive charge on the attached benzene ring. The effect is greatest at the ortho and para positions.
3rd group: The pair of electrons on the atom directly attached to the ring stabilizes a positive charge on the attached benzene ring. The effect is greatest at the ortho and para positions.
4th group: Halogens are peculiar in that they are o,p-directing yet deactivating.
Draw the major product(s) of nitration of nitrobenzene. If there is more than one product draw all in the same window.
Nitration: addition of NO2 . HOWEVER, there's already a substituent (NO2) so check the table. Table says that it should have a meta orientation so draw a 1,3 dinitrobenzene
Rank the compounds in each group according to their reactivity toward electrophilic substitution of bromine (bromination)
increased reactivity = decreased stability
a. check the position of the bromine: where would the present substitutent direct it to go?
b. check the position of the + charge:
-if it's right next to a deactivating group, then this makes the compound EXTREMELY UNSTABLE. This automatically makes it the least stable
c. check the carbon that the + charge is on:
- tertiary > secondary > primary in terms of stability of that carbocation
Draw the major monoalkylation product(s) you would expect to obtain from reaction of 2,4-dichloronitrobenzene with chloromethane and AlCl3. If there is more than one product draw all in the same window.
The ring is too deactivated to undergo Friedel-Crafts alkylation.
There are a few requirements for aromatic compounds undergoing Friedel-Crafts alkylations:
only alkyl halides can be used, not aryl halides or vinyl halides
2) can't have these substituents: NR3, NO2, CN, SO3H. CHO, CHOCH3, CO2H, NH2, CO2CH3, NHR, NR2. They deactivate the ring too much.
3) usually leads to polyalkylation (2 substitution reactions)
4) carbocation will rearrange to go from primary to a secondary carbocation (more stable)
Rank each of the following compounds in decreasing order of reactivity towards Friedel-Crafts alkylation.
Predict the major product(s) you would obtain from sulfonation of m-xylene.
Draw m-xylene
Know that sulfonation adds an SOOOH
Look and see if there's another substituent. If so, then you need to decide whether the positioning of the sulfur substituent is meta or o/p orientation relative to that substituent.
O/P-> two major products
M->one major product
Already two substituents?
Look at where those substituents usually direct other substituents
Look at the strength of reactivity of each substituent and see if they compete or compromise on one major product. Draw that.
Rank each of the following compounds in decreasing order of reactivity towards Friedel-Crafts alkylation.
Most reactive = 1; if a compound will not react, rank it as "non" rather than assigning a numerical value.
1) Highest reactivity-> ortho and para directing activators
then
Benzene
then
Ortho and para directing deactivators
then
Meta directing deactivators
2) Can't have these substituents: NR3, NO2, CN, SO3H. CHO, CHOCH3, CO2H, NH2, CO2CH3, NHR, NR2. They deactivate the ring too much.
No Friedel-Crafts alkylation will occur with those substituents.
Electrophilic substitution on 3-phenylpropenenitrile occurs at the meta position. Draw resonance structures to show how the ring is electron-poor at the ortho and para positions.
Use the + and - tools to adjust charges on the appropriate atoms.
The -CN group interacts with the ring through the π electrons of the side chain, deactivating the ring towards electrophilic substitution and directing substitution to the meta position.
Hydrogen Halide Addition to Conjugated Dienes
Draw both resonance structures of the most stable carbocation intermediate in the reaction shown. Do not include the halide anion.
Let MarvinSketch adjust the number of hydrogens automatically as you use the charge tool to specify formal charge . (Chapter 14; benzene plus HCl)
STRATEGY:
1) protonate 1 out of 2 terminal carbons (1 or 4) of the diene to make an allylic carbocation
2) Balance the charge and add double bonds where necessary (there will always be 1)
3) If the diene is asymmetrical, draw the other resonance structure.
Determine which set of resonance structures (1,4 adduct or 1,2 adduct) forms more stable carbocations
--> 3 degree > 2 degree > 1 degree
Draw the major 1,2- and 1,4-addition products obtained in the reaction shown. Assume that both are derived from the most stable carbocation intermediate.
This is the same strategy for the previous question EXCEPT instead of choosing the two resonance structures that are more stable, you're picking the most stable resonance structure from a 1,2 addition and a 1,4 addition. You'll then need to draw the figures.
ADD IN THE HALIDE TO THE CARBOCATION.
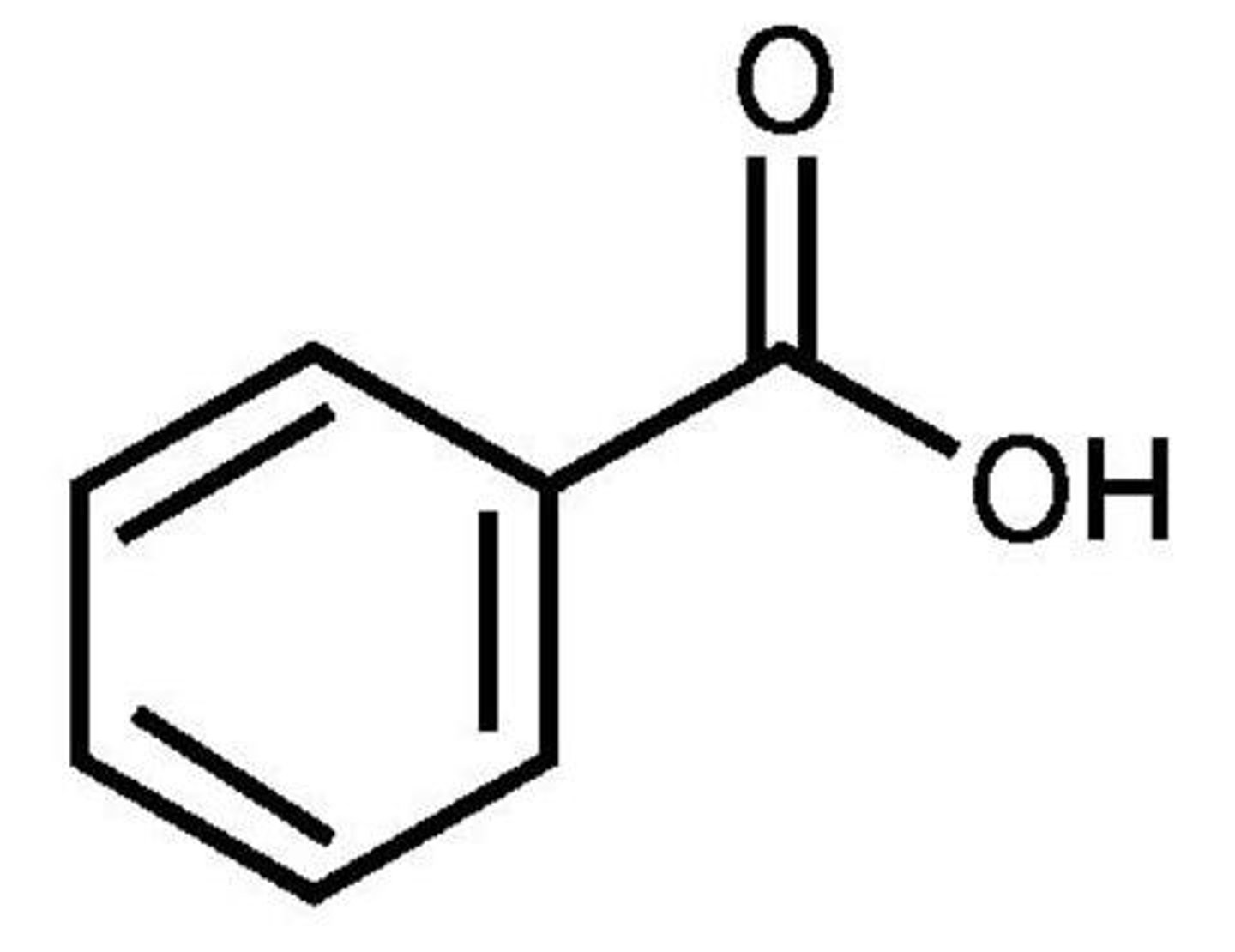
benzoic acid
carboxylic acid + benzene
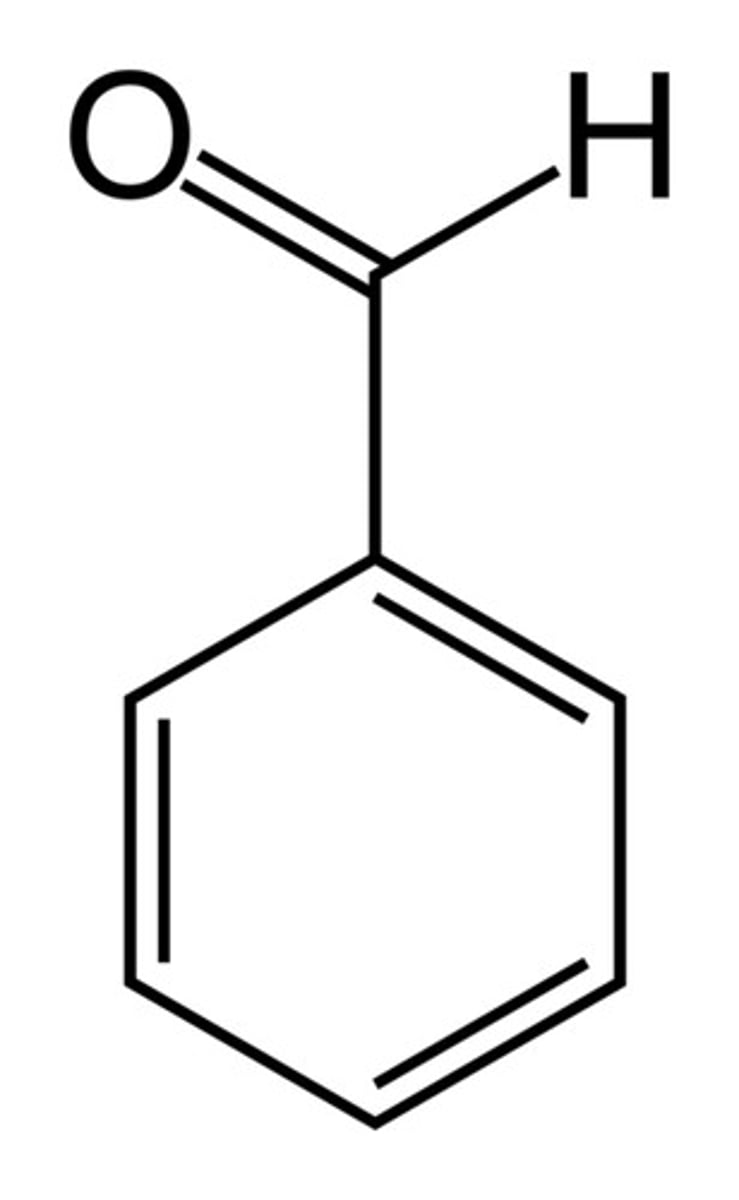
benzaldehyde
aldehyde + benzene
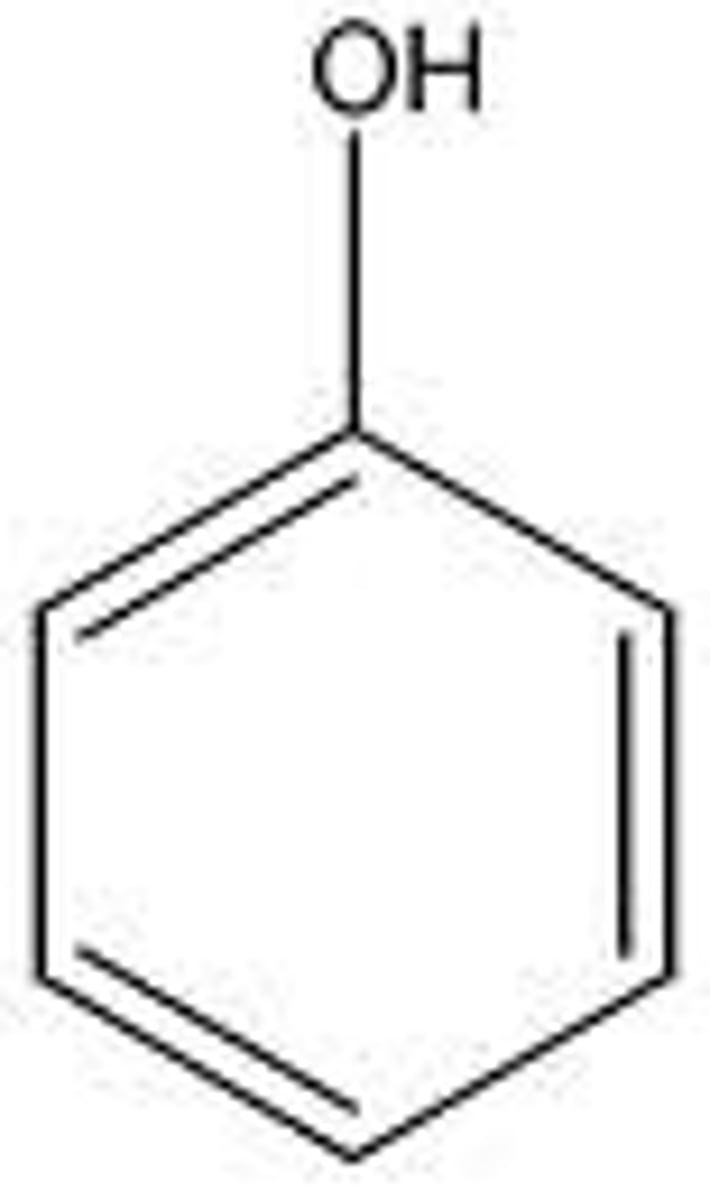
phenol
alcohol + benzene
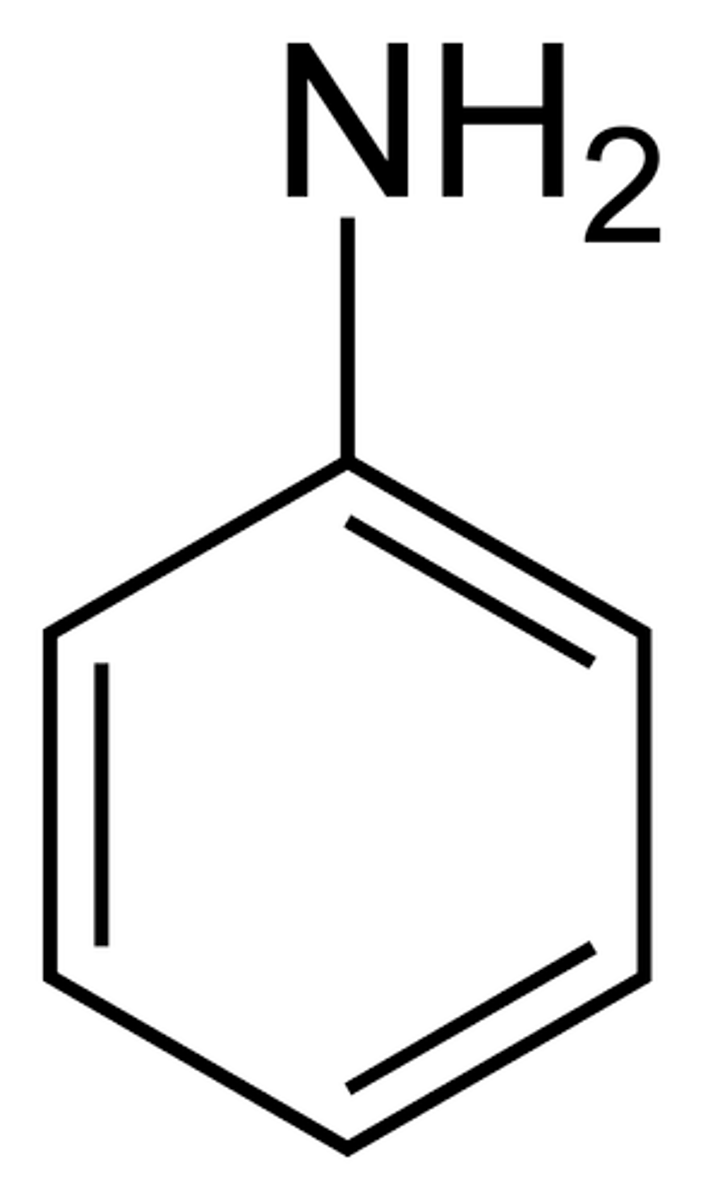
aniline
amine group + benzene
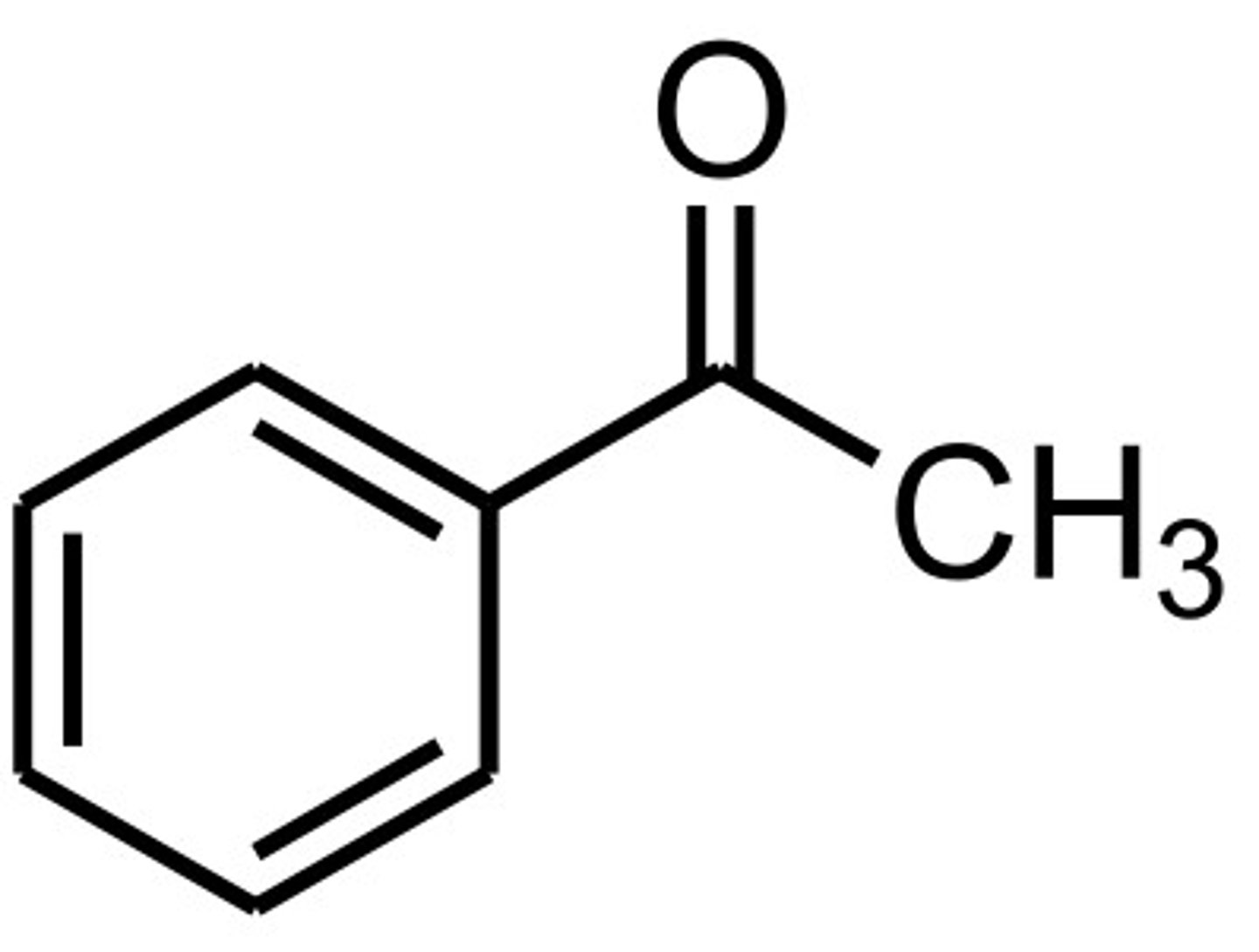
acetophenone
ketone + benzene
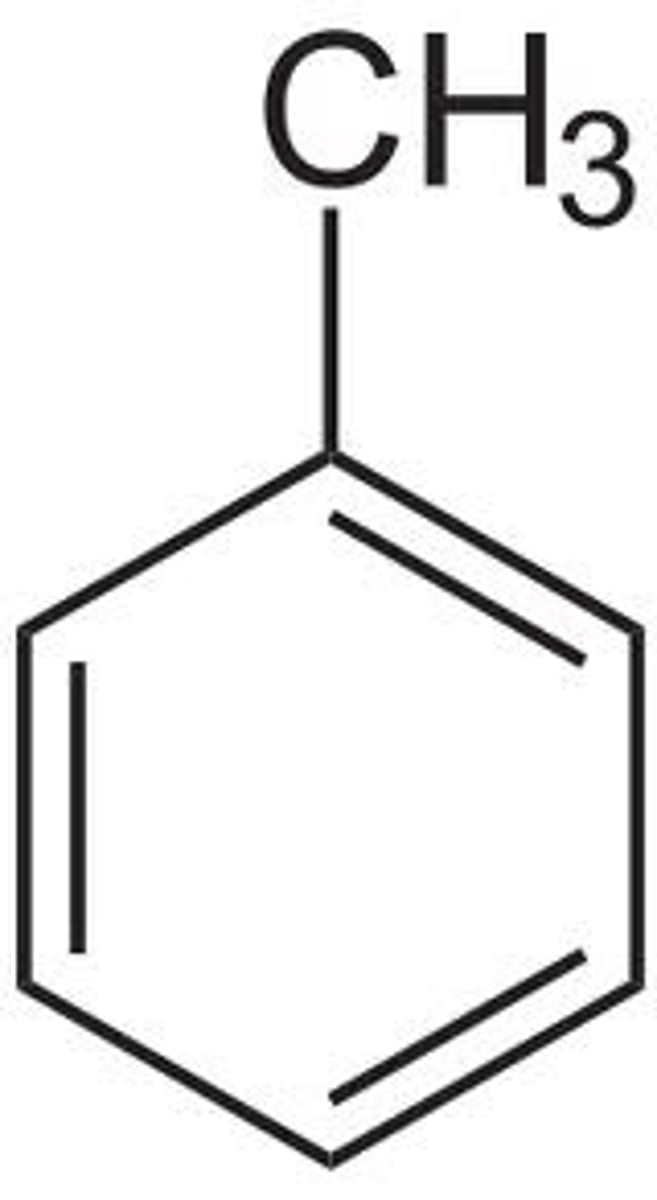
toluene
methyl + benzene
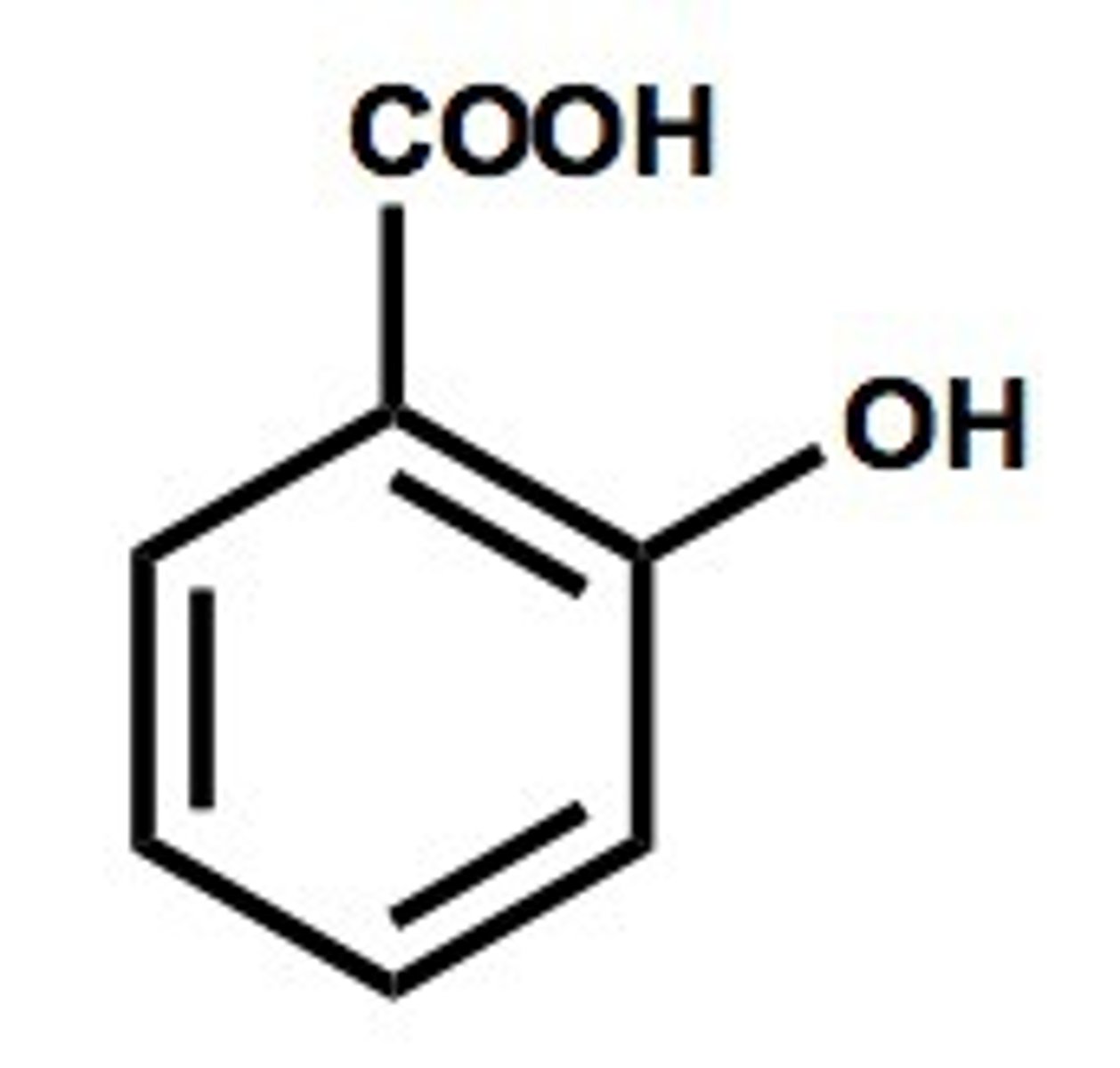
2-hydroxy benzoic acid
carboxylic acid + an alcohol group tacked on to a benzene
Benzene nomenclature
1) Mono-substituted rings do not need numbers
2) Di-substituted rings: o, m, p orientation
--> < 6 carbons: remains a substituent
--> 7 > carbons: benzene becomes the substituent
3) Tri-substituted rings:
--> number the location of each substituent starting at the lowest locate number at the first point of difference
--> HOWEVER, numbering starts at the principal functional group if it determines the base name
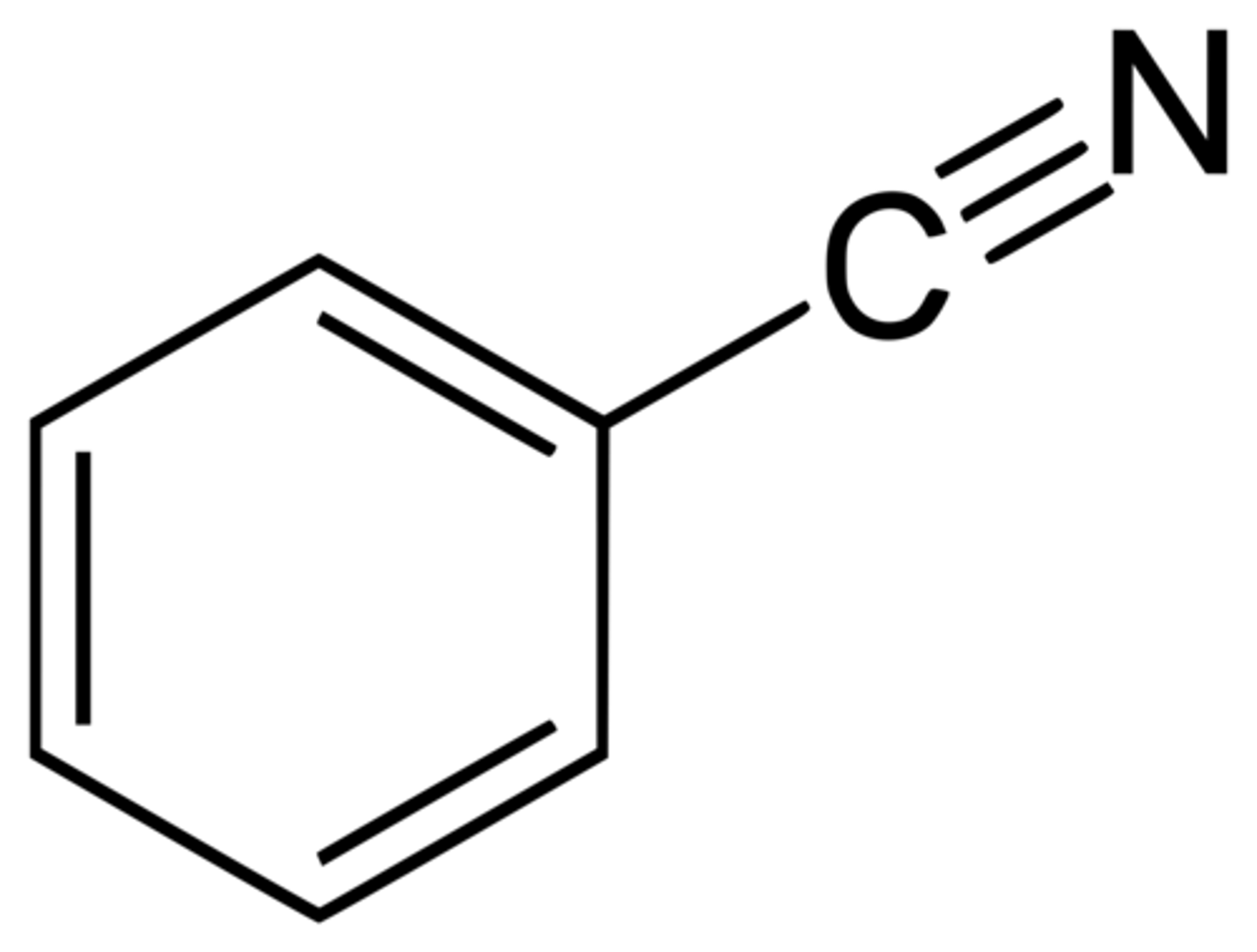
benzonitrile
CN + a benzene
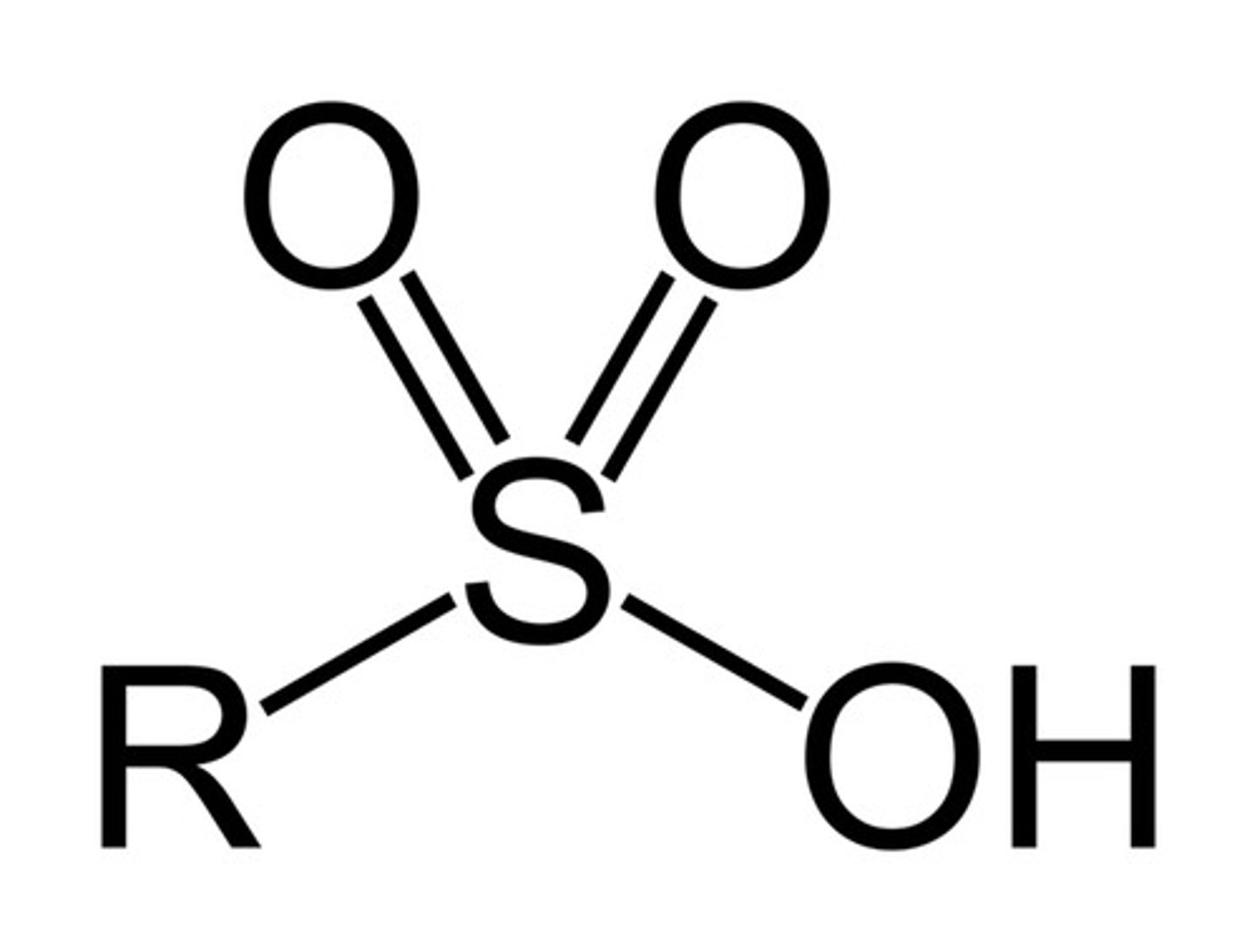
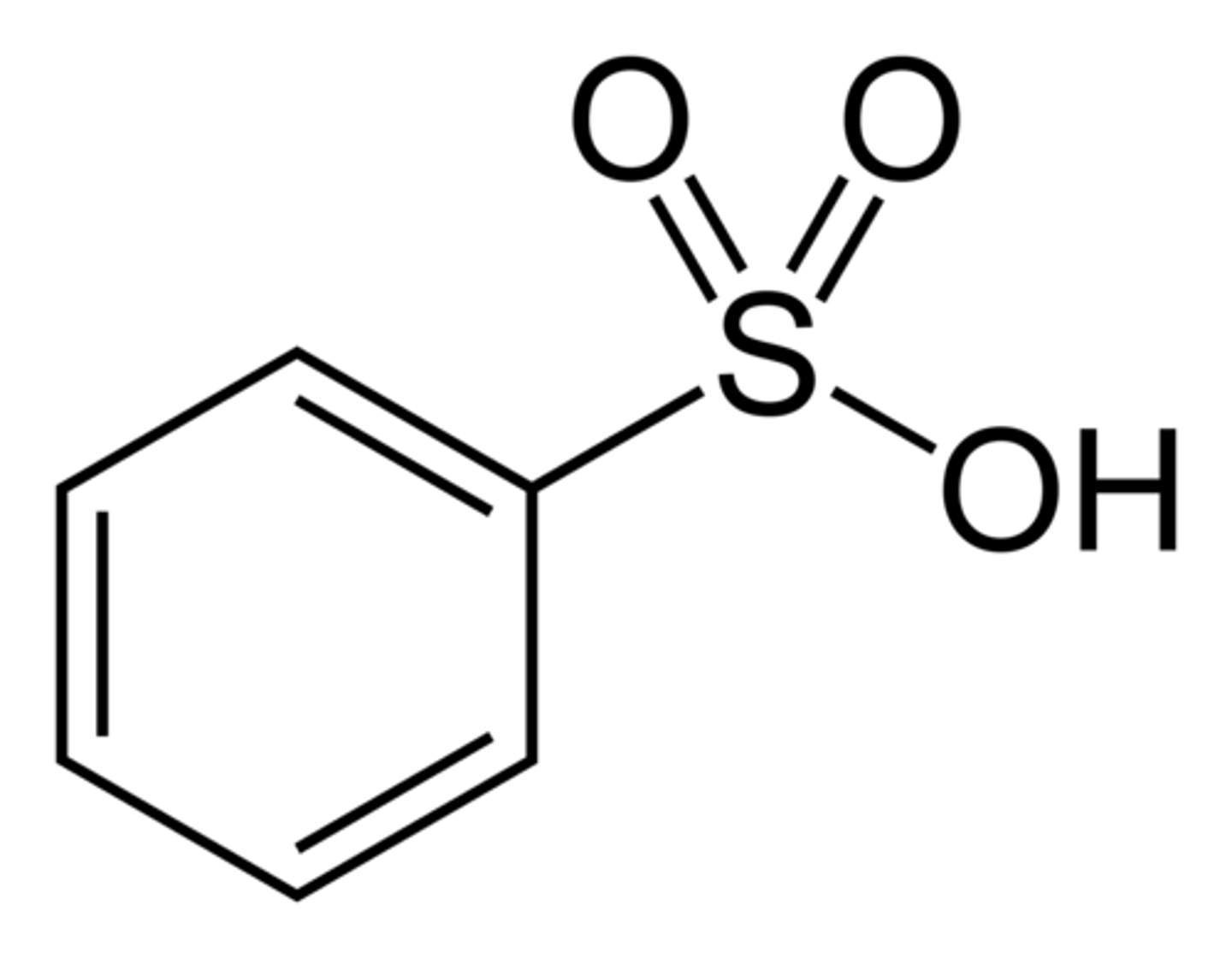
benzenesulfonic acid
SO3H + a benzene
For each of the species below, identify any cyclic conjugated system, then:
A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation).
B. Specify whether the species is "a"-aromatic, "aa"-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic).
(Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count electrons in the largest.)
Rules of Aromaticity:
1) is it conjugated? (alternating single and double bonds)
2) is it cyclic?
If the answer to one of these q's is no, then it's non-aromatic:
--> discontinuous, conjugation is interrupted by an sp3 carbon or there's a change in planarity of the p-orbitals
--> cross conjugation: alkene is bonded to middle atoms of a conjugated ring through single bonds
If it's conjugated and cyclic and planar, then is it:
Aromatic:
--> cyclic continuous conjugated double bonds
--> planar
--> electrons in the conjugated system follow the 4N +2 rule
Anti-aromatic:
--> cyclic continuous conjugated double bonds
--> planar
--> electrons in the conjugated system follow the 4N rule
Considerations:
--> (N, O, S) involved in a double bond: double bond = 1 and N = 1 electron
--> (N, O, S) involved in single bonds: both LPEs are in cyclic conjugation
--> O with 4 electrons donates 2
--> poly-cyclic aromatic compounds have shared rings that all
Charged carbon:
--> negative: donates 2 e's
--> positive: donates no e's
NMR
peaks --> types of hydrogens in molecule
chemical shift--> where they appear in the shift determines what they are attached to
--> H's attached to electron withdrawing groups appear to be (downfield)
integration--> relative area will tell you how many H's gave rise to that peak
spin-spin splitting--> looks at environment of each proton then uses the n+1 rule to determine the number of splits within each peak.
triplet or quartet is typically a CH3CH2 part of the molecule.
Predict the splitting patten for each of the labeled hydrogens in the following molecules
1) Look at the first proton
2) Check the carbons that neighbor the carbon it's attached t
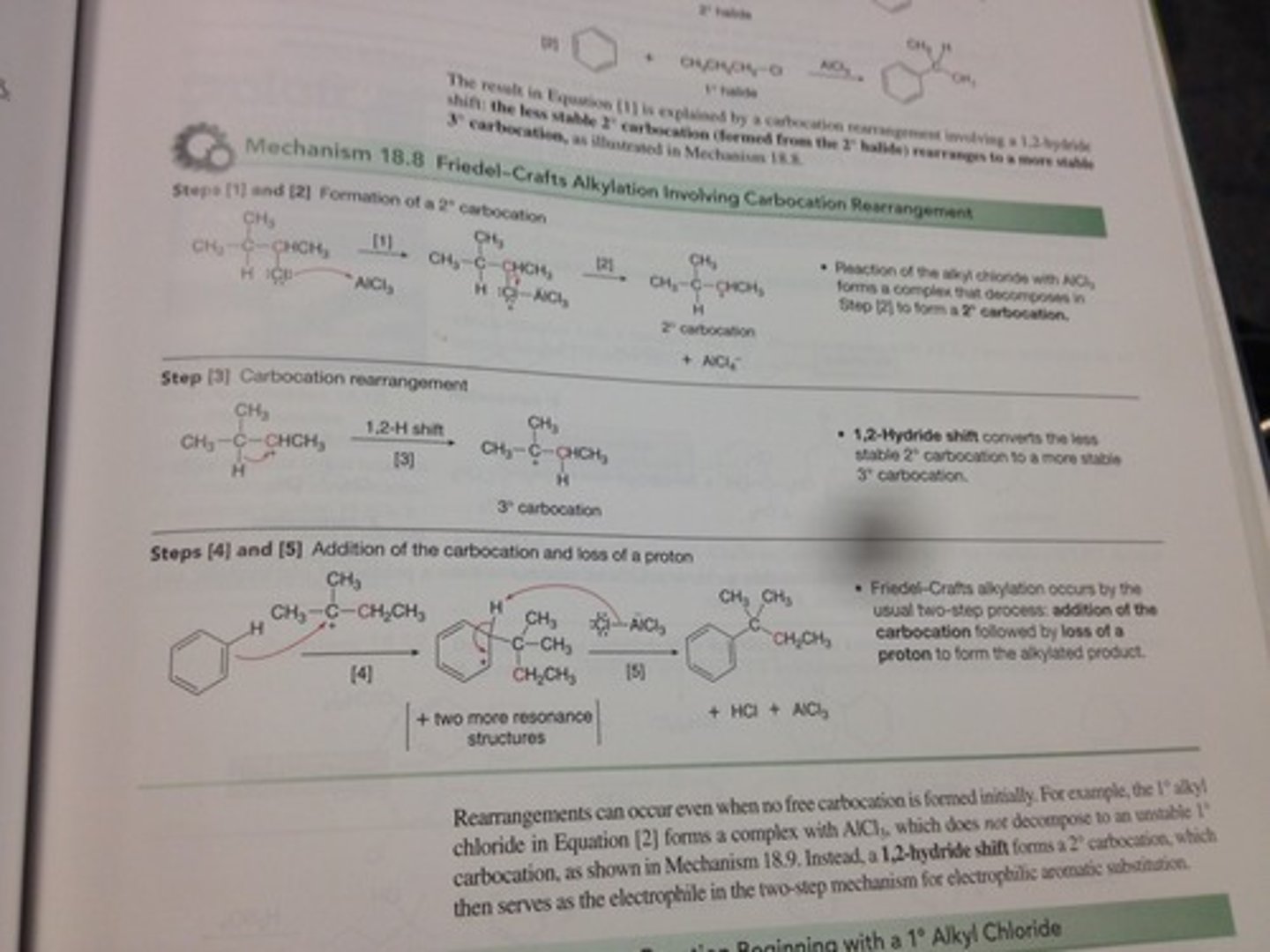
Limitations of Friedel-Crafts Alkylation
Requirements for these reactions to occur:
-aromatic ring must be at least as reactive as benzene
-alkyl halide - primary alkyl halides undergo carbocation rearrangement
lkylation:
1) ONLY alkyl halides can be used NOT aryl halides or vinyl halides
2) Can't have these substituents: NR3, NO2, CN, SO3H, CHO, COCH3, CO2H, NH2, CO2CH3, NHR and NR2. These are all deactivating substituents so they won't allow the reaction to take place.
3) Usually leads to polyalkylation (substitute 2 groups in para position) so you can't just stop with one substituent.
4) carbocation will rearrange to go from being primary to secondary due to it being more stable as a secondary carbocation.
Acylation:
1)Can never have more than 1 substituent
2)Can't have those deactivating substituents on the benzene ring already.
Select the intermediate in the bromination of phenol?
This is an electrophilic aromatic substitution reaction:
1) generate the electrophile:
Mechanism of Freidel-Crafts Alkylation and Acylation:
Alkylation:
1) CH3Cl OR COR group reacts with AlCl3 to make an electrophilic carbocation, R+
2) Electron pair from the aromatic ring attacks carbocation, forming new bond. Carbocation intermediate is formed.
3) AlCl4- takes away an H+ to leave a substitution product.
Relate Conjugation to Energy, Wavelength and Stability
increase the degree of conjugation = decreased difference between HOMO and LUMO = decreased energy = increased wavelength
Look at the molecules:
no resonance (pi bonds are separated more than a carbon away) = less stable
more substituted pi bond = more stable
Which of the following molecules fit the chemical shift display shown above?
1) Look at the integral ratio: this is the sum of the protons
-if it matches the molecular formula, then this is the number of hydrogens in each type
2) Look at where the peaks are on the chemical shift chart
-benzene is 6-8 ppm
3) Look at the spin-spin splitting pattern in peaks:
This will tell you how many H's that hydrogen is next to
Follows n + 1 rule
Rank parent names of benzene compounds
The lowest number group will win the parent name
1) benzoic acid
2) benzaldehyde
3) acetophenone
4) phenol
5) aniline
6) toluene