Chemistry OCR - Module 4: Section 2 Alcohols, Haloalkanes & Analysis
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What is a haloalkane?
An alkane with at least on halogen atom
Why is the Carbon-Halogen bond in haloalkanes polar?
Halogens are more electronegative than carbon, so the carbon-halogen bond is polar.
The δ+ carbon is electron deficient. This means it can be attacked by a nucleophile.
What is a nucleophile?
A nucleophile is an electron pair donor. It could be a negative ion or an atom with a lone pair of electrons. It donates an electron pair to somewhere without enough electrons.
OH-, CN-, and NH3 are all nucleophiles which react with haloalkanes. Water is a nucleophile too, but it reacts slowly.
How can haloalkanes be hydrolysed to make alcohols?
This is a nucleophilic substitution reaction. You have to use a warm aqueous alkali, for example sodium hydroxide, or potassium hydroxide or it will not work. The general equation is: R-X + OH- → R-OH + X-
(over the arrow is OH-/H2O and below is reflux)
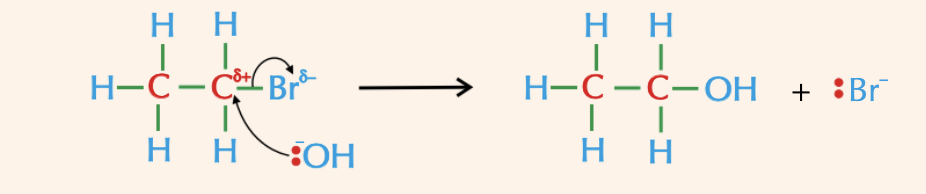
Why can water act as a nucleophile?
The water molecule is a weak nucleophile, but it will eventually substitute for the halogen, it is just a much slower reaction than one with a halogen.
You get an alcohol produced again: R-X + H2O → R-OH + H+ + X-
Why are iodoalkanes hydrolysed the fastest?
How quickly different haloalkanes are hydrolysed depends on bond enthalpy.
Weaker carbon-halogen bonds break more easily so they react faster.
Iodoalkanes have the weakest bonds, so they hydrolyse the fastest.
Fluoroalkanes have the strongest bonds, so they are the slowest at hydrolysing.
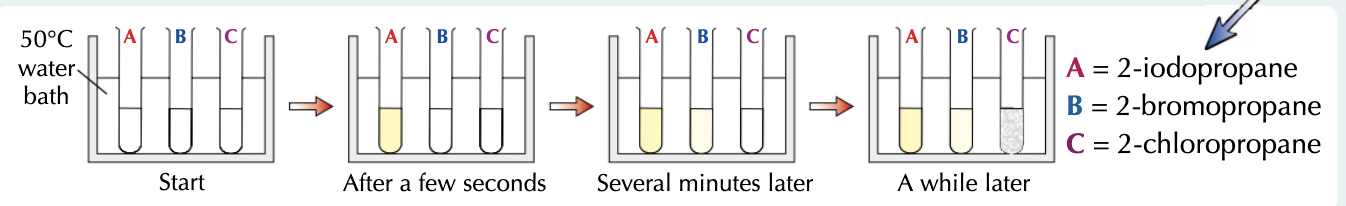
How can you compare the reactivity of chloroalkanes, bromoalkanes and iodoalkanes?
When you mix a haloalkane with water, it reacts to form an alcohol.
If you put silver nitrate solution in the mixture too, the silver ions react with the halide ions as soon as they form, giving a silver halide precipitate.
To compare the reactivities, set up three test tubes each containing a different haloalkane, ethanol (as a solvent) and silver nitrate solution (this contains the water).
A pale yellow precipitate quickly forms with 2-iodopropane, so iodoalkanes must be the most reactive haloalkanes. Bromoalkanes react slower than iodoalkanes to form a cream precipitate, and chloroalkanes form a white precipitate the slowest of all.
What are CFCs?
Chlorofluorocarbons
They are very stable, volatile, non-flammable and non-toxic. They were used a lot, e.g. in fridges, aerosol cans, dry cleaning and air conditioning, until scientists realised they were destroying the ozone layer.
Why are CFCs destroying the Ozone layer?
The ozone absorbs UV radiation which can cause sunburns or skin cancer.
Ozone formed naturally, when an oxygen molecule is broken down into two free radicals by ultraviolet radiation. The free radicals attack other oxygen molecules forming ozone: O2 → O + O → O2 + O → O3
The holes in the ozone are formed because CFC’s in the upper atmosphere absorb UV radiation and split to form chlorine free radicals. These free radicals catalyse the destruction of ozone, they destroy ozone molecules and are then regenerated to destroy more ozone.
CF2Cl2(g) → ·CF2Cl(g) + Cl·(g)
Cl·(g) + O3(g) → O2(g) + ClO·(g)
ClO·(g) + O(g) → O2(g) + Cl·(g)
Overall: O3(g) + O(g) → 2O2(g)
How can nitrogen oxides also break down ozone?
NO· free radicals from nitrogen oxides destroy ozone too. Nitrogen oxides are produced by car and aircraft engines and thunderstorms. NO· free radicals affect ozone in the same way as chlorine radicals.
What alternatives have been developed for CFCs?
HCFCs (hydrogchlorofluorocarbons) and HFCs (hydrofluorocarbons) are being used as temporary alternatives to CFCs until safer products are developed.
Hydrocarbons are also used.
HCFCs are broken down in the atmosphere in 10-20 years. The still damage the ozone layer, but their effect is much smaller than CFCs.
HFCs are also broken down in the atmosphere. Unlike HCFCs, they do not contain chlorine, so they do not affect the ozone layer.
HFCs and HCFCs are greenhouses gases and they are 1000 times worse than carbon dioxide.
Some hydrocarbons are being used in fridges but these are greenhouse gases too.
Most aerosols have been replaced by pump spray systems or use nitrogen as a propellant. Many industrial fridges and freezers now use ammonia as the coolant gas, and carbon dioxide is used to make foamed polymers.