P1 Booklet 1- Density
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Density
The mass per unit volume of a material
Density equation
D = M/V
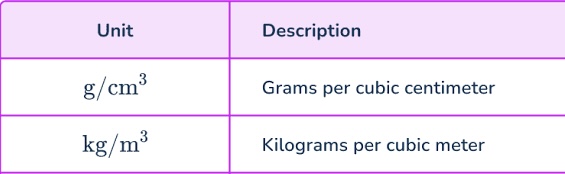
Units for density
Kg/m3 and g/cm3
Density description
Solids are more dense than liquids.
Liquids are more dense than gases.
This is because the particles are more tightly packed.
Mass
Mass is the amount of matter a subject contains. (Mass of an object remains constant - it doesn’t change)
Apparatus for working out mass.
Digital balance.

Units for mass
Grams (g) & Kilograms (kg)
Volume
The amount of space an object or substance occupies.
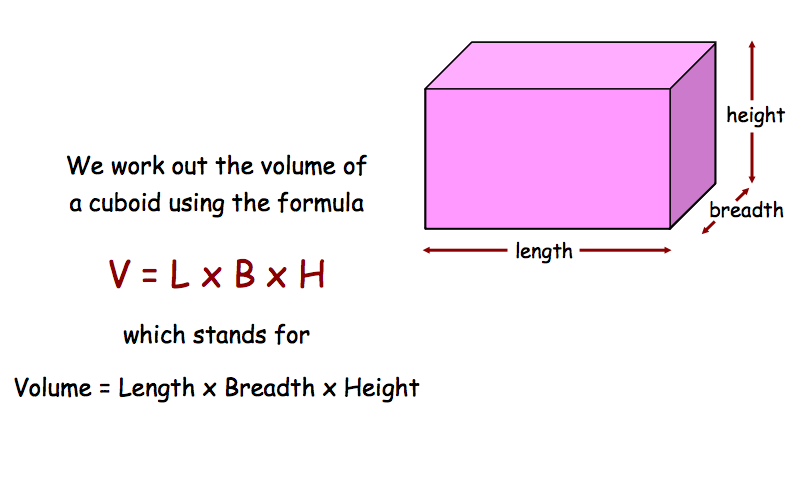
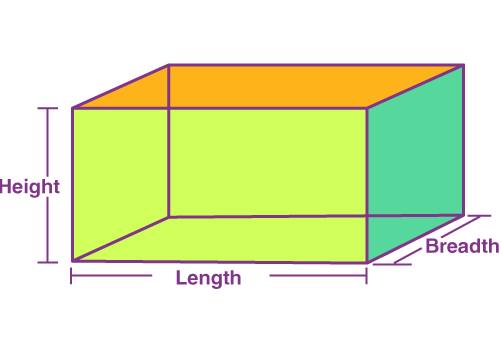
Equation for volume.
V = l x b x h
What is volume measured in?
mm³ or cm³ or m³
What are the units of volume?
l or ml
Finding the density of a regular solid.
1.) Measure and record the dimensions/lengths of each side of a cuboid using a ruler.
2.) Calculate its volume using
V = l x b x h
3.) Place each cube/cuboid on the balance and record its mass.
4.) Calculate the density using d = m/v
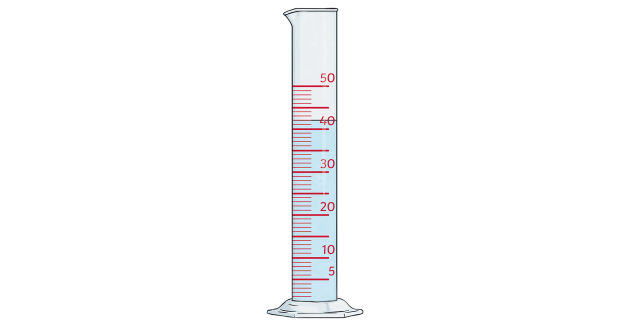
Finding the density of an irregular solid using a measuring cylinder.
1.) Place a number of coins/rivets on the digital balance and record their total mass.
2.) Put some water in a measuring cylinder.
3.) Read V1 before object is added and V2 when object is added.
4.) Volume of object = V2 - V1
5.) Calculate density using d = m/v
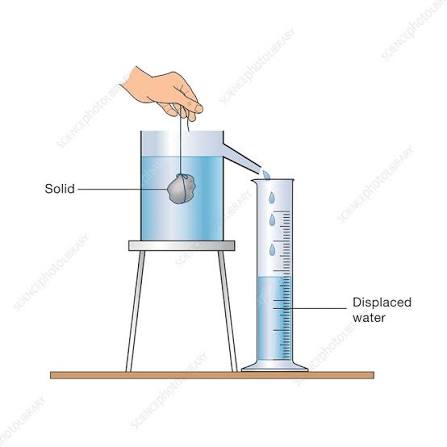
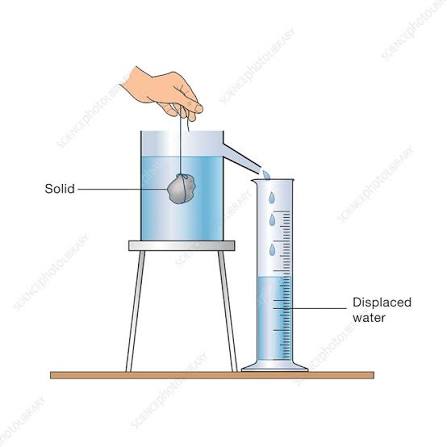
Finding the density of an irregular solid using the displacement can.
1.) Place each stone on the balance and record its mass.
2.) Fill the displacement can until it is about to overflow. Add the object.
3.) Measure the volume of each object by measuring the volume of water displaced into the measuring cylinder.
4.) Calculate density using d = m/v
Finding the density of a liquid.
1.) Place a 100ml measuring cylinder onto a digital balance.
2.) Find the mass of the empty cylinder.
3.) Pour 100cm3 of water into the cylinder and record its mass and volume.
4.) Mass of the liquid = mass of cylinder with liquid - mass of cylinder.
5.) Calculate density using d=m/v