General Chemistry 1 Final
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
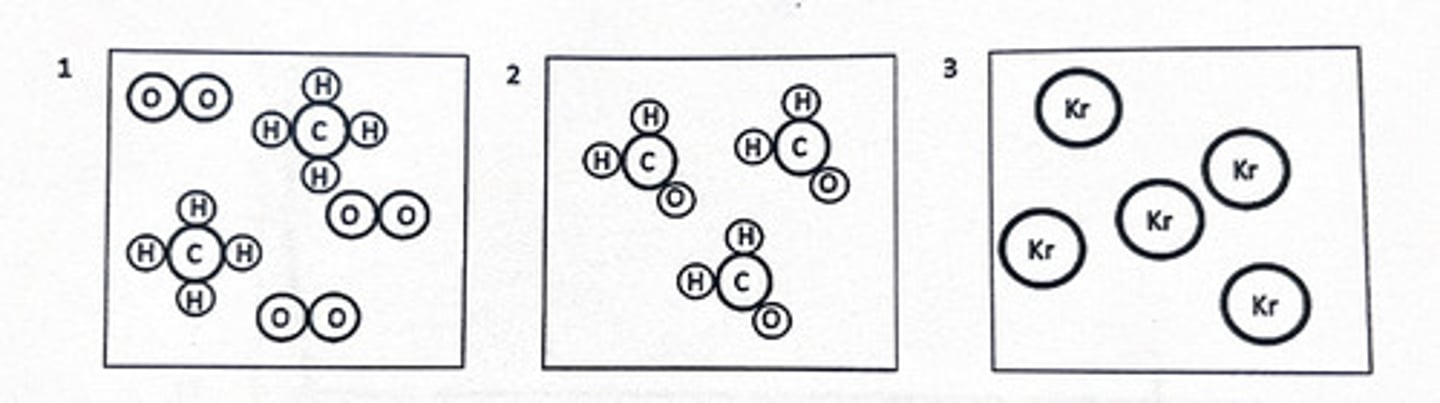
1 and 3
Determine which statement(s) is/are true based on the figures below:
1) Image 1 is a representative of a mixture of 2 different molecules
2) Image 2 is a representative of a mixture of atoms
3) Image 3 is a representative of a pure substance
1 and 2
What statement(s) is/are true about the following particles?
1) Particle 1 is a compound
2) Particle 2 is an atom of an element
3) Particle 3 is a compound

E) None of them are true.
Which of the following is true about scientific theories?
A) A scientific theory cannot be falsified
B) A scientific theory is a possible explanation with little or no supporting evidence
C) A scientific theory describes a phenomenon
D) All of them are true
E) None of them are true.
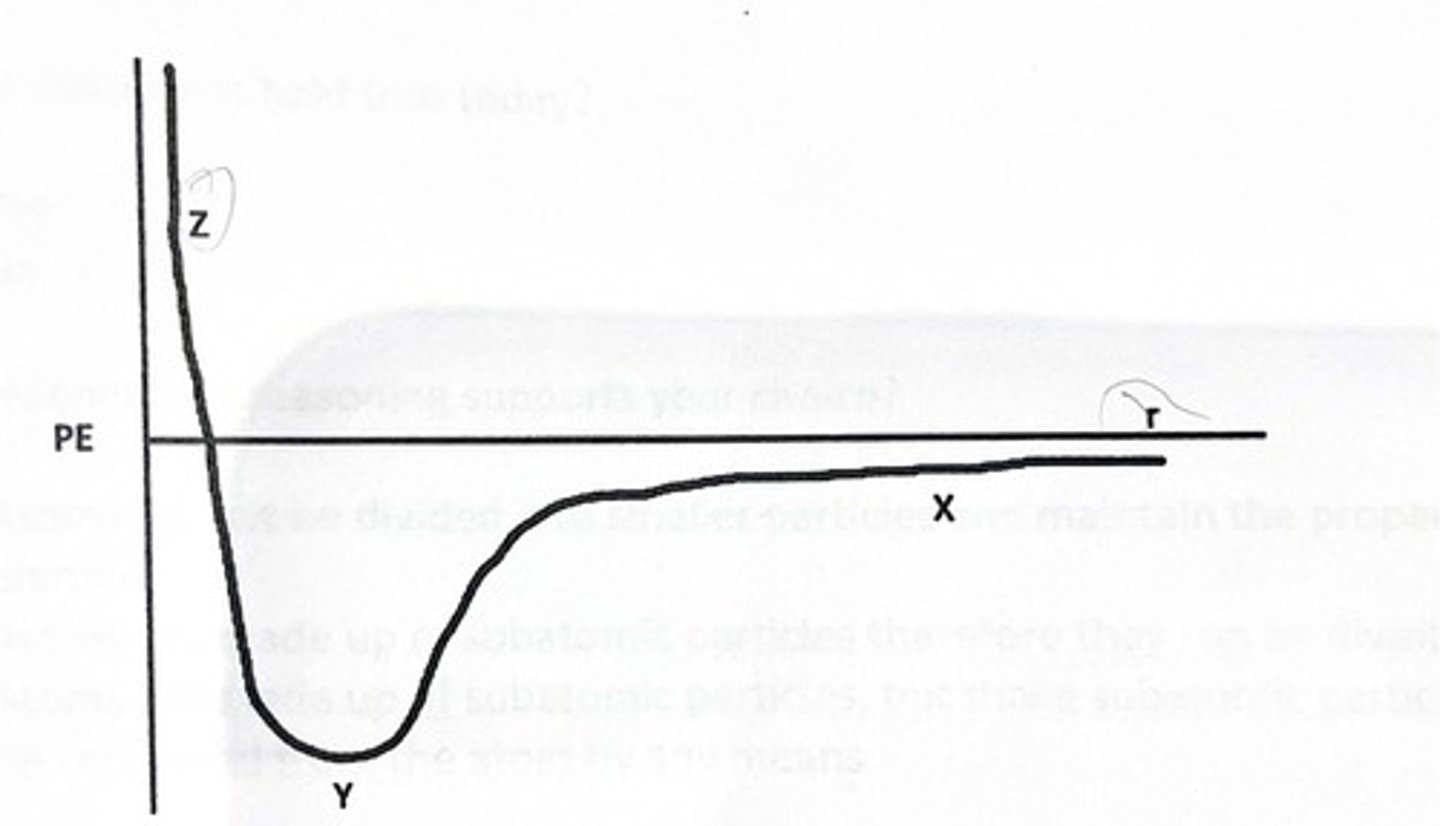
1 and 5
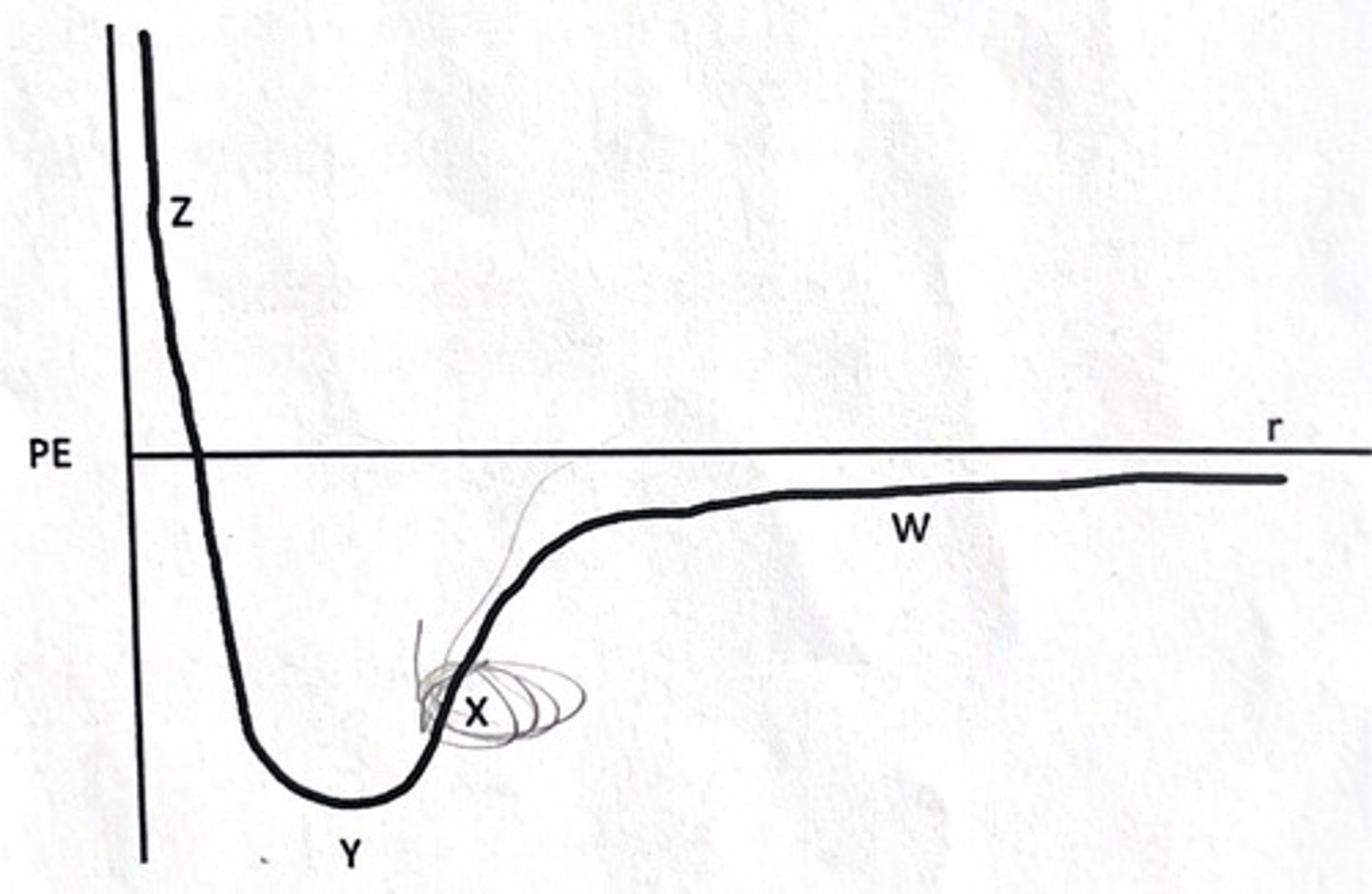
The graph below shows the change in potential energy as the distance between two neon (Ne) atoms changes.
What is true about the forces at point Z?
1) The repulsive force is greater than the attractive force
2) The attractive force is greater than the repulsive force
3) The attractive and repulsive forces are equal
How were you able to make this determination?
4) The total energy is at its highest
5) The potential energy is at its highest
6) The potential energy is at its lowest
C. All atoms of an element are identical and have the same mass and properties
Which of the following statements about Dalton's atomic theory is false?
A. Atoms of a given element are different from atoms of another element for example they have a different number of protons
B. Atoms of an elements are not identical because different isotopes have different masses
C. All atoms of an element are identical and have the same mass and properties
D. Compounds are formed from a combination of atoms of two or more different elements
E. Matter is neither created nor destroyed in a reaction
2 and 4
Part of Dalton's atomic theory stated " elements are composed of small indestructible particles called atoms."
Does this statement hold true today?
1) Yes
2) No
What evidence and reasoning supports your choice?
3) Atoms cannot be divided into smaller particles and maintain the property of the element
4) Atoms are made up of subatomic particles therefore they can be divisible
5) Atoms are made up of subatomic particles, but those subatomic particles cannot be separated from the atom by any means
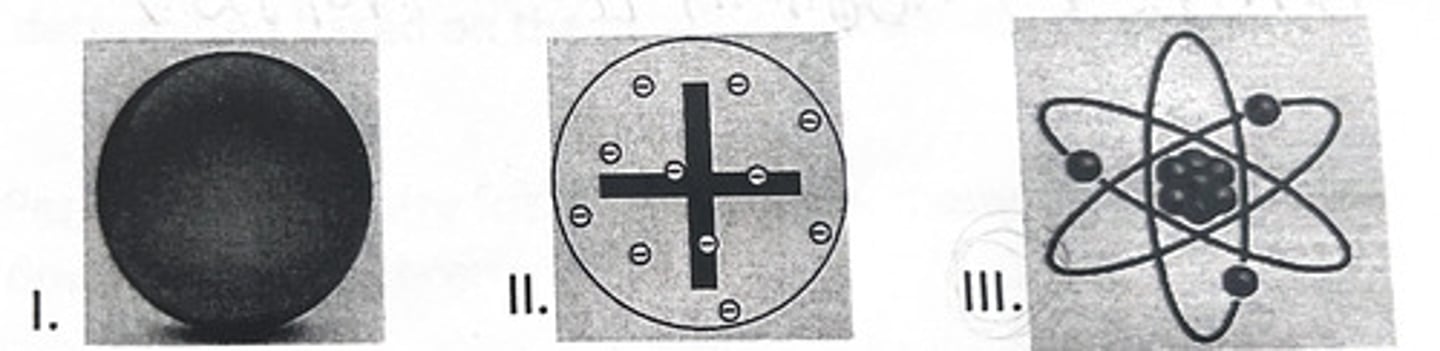
III and V
Which of the following models best represents the model of the atom proposed by Rutherford and why?
Because:
IV. according to Rutherford the atom was an indivisible and indestructible particle.
V. according to Rutherford the atom contains a very small, dense, and positively charged nucleus.
VI. according to Rutherford the atom contains negatively charged particles embedded in a positively charged body.
1 and 3
During class, we discussed gravitational and electrostatic forces. Which of the following statements are false about the two forces?
1) Both forces can be attractive or repulsive depending on the masses and charges involved.
2) As the distance between two object with mass increases, the gravitational force decreases.
3) For gravitational force, the masses of the objects are directly proportional to the repulsive force.
4) For electrostatic force, two objects with opposite charges are attracted to each other and this attractive force increases as the changes increase.
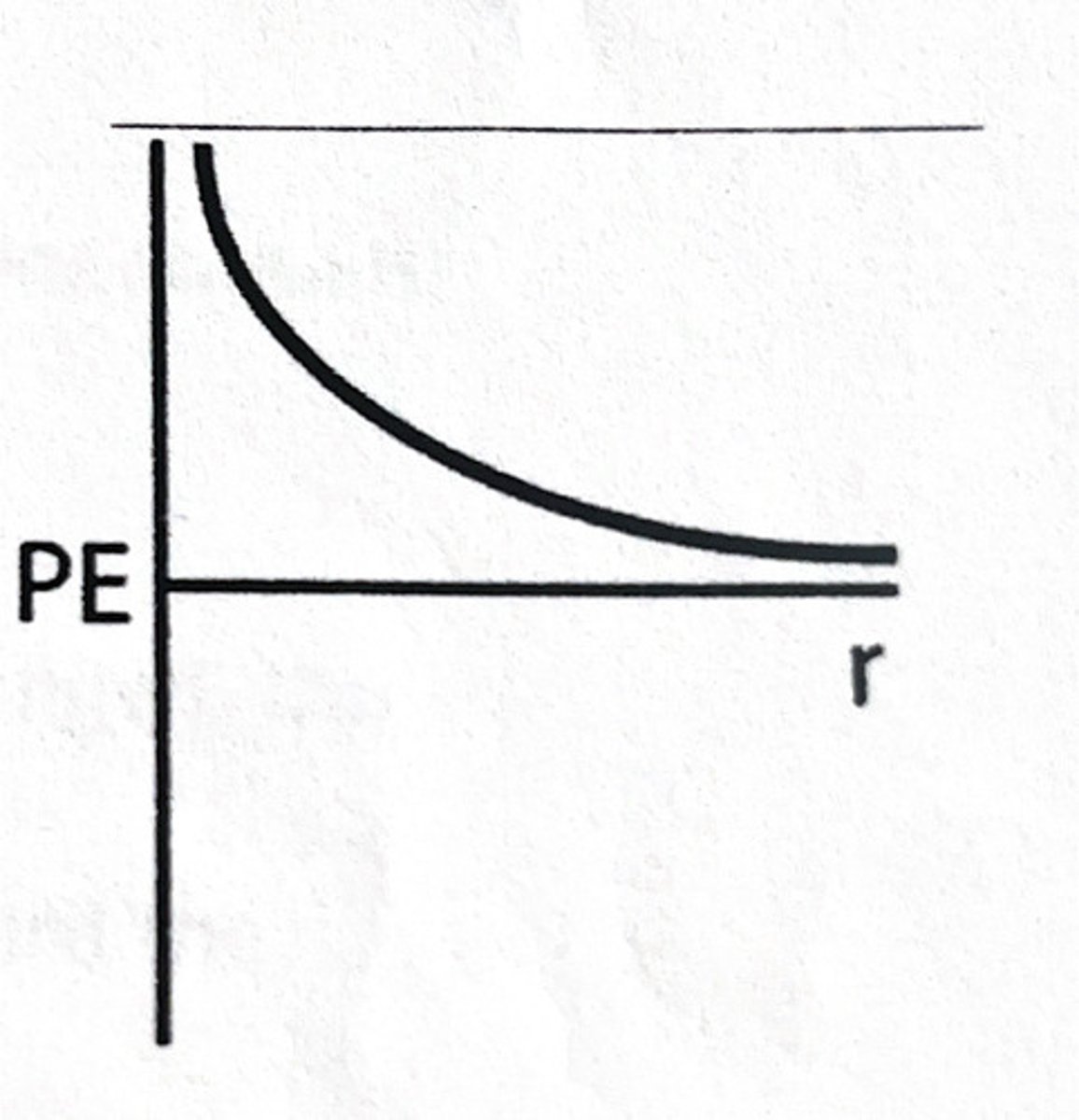
B
Which of the following scenarios would result in the potential energy curve shown here?
A) Two objects with mass approaching each other.
B) Two charged particles with the same charge approaching each other.
C) Two charged particles with opposite charges approaching each other.
D) Two atoms approaching each other.
E) Cannot be determined based on the provided information.
35 protons and 35 electrons
At right is the periodic table entry for bromine. How many protons and electrons does an atom of bromine have?

B
Which of the following is the best example of a scientific question?
A) Ghosts are real.
B) Which liquid helps plants grow faster, water or coffee?
C) The best tasting Starburst flavor are the pink ones (strawberry)
D. What is your favorite Halloween candy?
1 and 3
Imagine an isolated system where Katie drops two balls, red and blue, from the same height above the ground. The red ball has a mass of 50 grams and the blue ball has a mass of 100 g. Based on the provided information, which of the following statements are correct.?
1) The blue ball experienced a greater gravitational force than the red ball.
2) While the balls are falling, the only force acting on them is electromagnetic.
3) The potential energy decreases as the balls fall because the attractive force is increasing.
4) The increase in kinetic energy is caused by the decrease in potential energy .
5) The total energy of the system is increasing.
1 and 4
Identify the specific evidence and reasoning for the following claim: "All atoms contain electrons."
Evidence:
1) When the material from which the cathode is composed of is changed, the behavior of the beam of particles stays the same.
2) The beam of particles emerges from cathode and bends toward the positively charged plate.
Reasoning:
3) objects with opposite charges attract each other through the electrostatic force.
4) The particles emerge from atoms of all different elements.
D
Imagine a scenario where there is a soda can sitting on your dining room table. Why is the soda not moving?
A. There are no forces acting on the can.
B. The gravitational force is pulling the can toward the table.
C. The electrostatic force between the can and the table is repulsive.
D. The gravitational and electrostatic forces acting upon the can are equal and opposite.
1 and 6
What happens to the potential energy when two atoms approach and get so close that their electron clouds overlap?
The potential energy:
1) Increases
2) Decreases
3) Stays the same
Because:
4) This is an isolated system.
5) The kinetic energy decreases.
6) The electron clouds repel each other.
7) The London dispersion forces are very strong.
B
Substances are considered isotopes of each other when they have __ number of protons and __ number of neutrons.
A) Same, same
B) Same, different
C) Different, same
D) Different, different
A
Recall our class investigation of two helium atoms as they approach. Which of the following statements is true regarding the total energy of the isolated system of the two atoms?
A) The total energy of the system will remain constant, because energy cannot be created or destroyed.
B) The total energy of the system will decrease, because of the electrostatic electrostatic attraction between two atoms.
C) The total energy of the system will increase, because of the electrostatic repulsion between the two atoms.
D) The total energy of the system will decrease, because of the electrostatic repulsion between the two atoms.
E) The total energy of the system will increase, because the electrostatic repulsion between the two atoms.
1, 3, and 5
When two He atoms move toward each other with instantaneous dipoles, what happens to the kinetic energy of the system and why?
The kinetic energy:
1) Increases
2) Decreases
Because:
3) The force of attraction is stronger
4) The force of repulsion is stronger
5) The velocity of the atoms is increasing.
6) The velocity of the atoms is decreasing
D
Which of the following statements regarding LDFs is/are true?
A) LDFs are present between all atoms and molecules.
B) LDFs are caused by fluctuations in an atom's or molecule's electron cloud.
C) LDFs are and example of an intermolecular force.
D) All of these statements are true.
E) None of these are true
C
What can you say about point X on the graph?
A) The electrostatic repulsive force is greater than the electrostatic attractive force
B) The electrostatic attractive force is equal to the electrostatic repulsive force.
C) The electrostatic attractive force is greater than the electrostatic repulsive force
D) The total energy of the system is decreasing
E) The total energy of the system is increasing
2 and 6
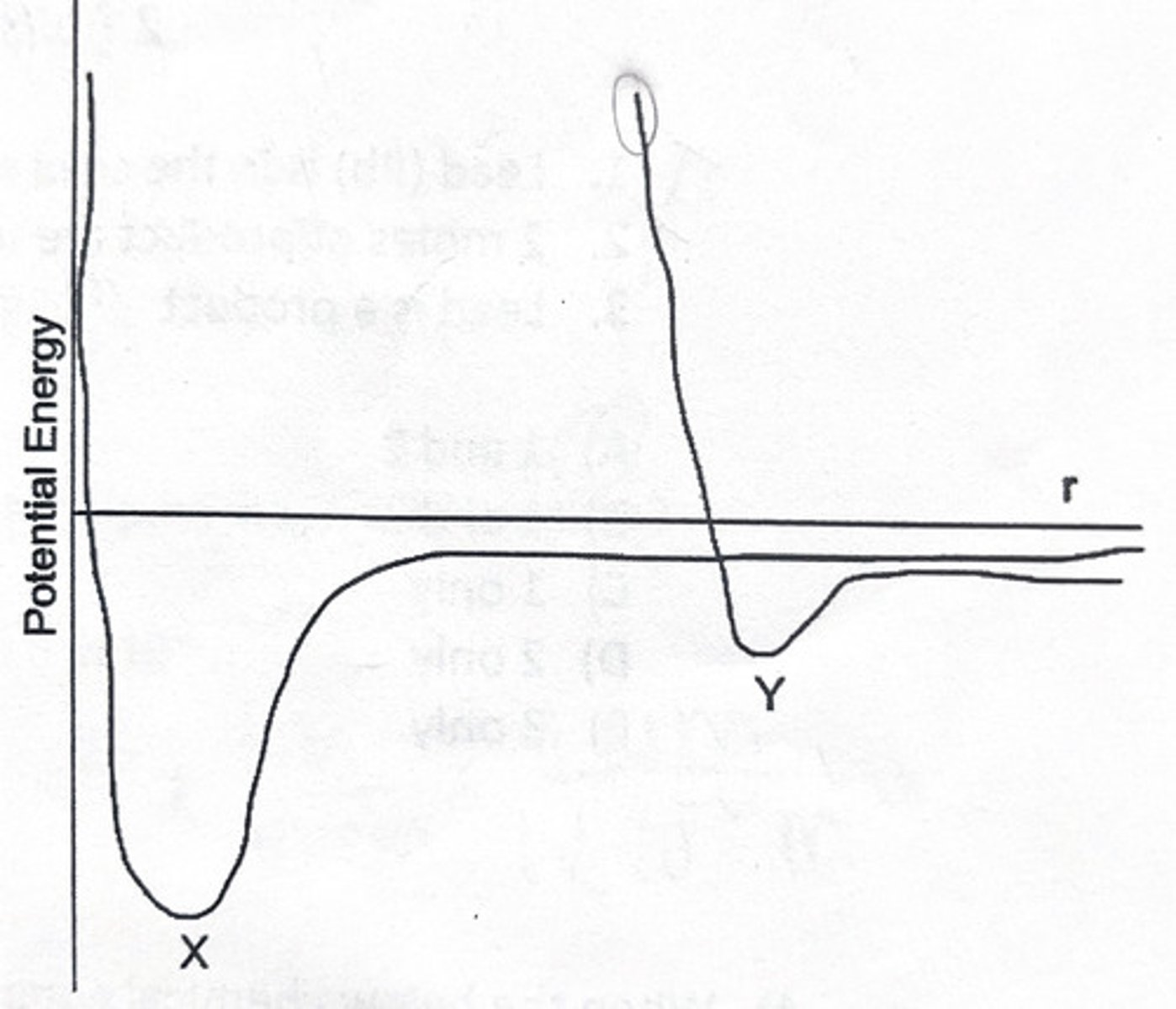
The below graph shows the change in potential energy as the distance between two He atoms and two H atoms changes. Which curve represents hydrogen?
1. Y
2. X
How did you determine which one was hydrogen over aluminum?
3. The atoms are closer together and the potential energy well is deeper for hydrogen because it has London dispersion forces.
5. The atoms are closer together and the potential energy well is deeper for helium because it has London dispersion forces.
6. The atoms are closer together and the potential energy well is deeper for hydrogen because it forms a covalent bond.
E
Which of the following would require the most energy to separate two of the atoms at the bottom of the potential energy minimum well?
A) He with He
B) Ar with Ar
C) Kr with Kr
D) Ne with Ne
E) Cl bonded to Cl
1 and 2
Which of the below statement are true about the following chemical equation?
2Pb (s) + O2 (g) -> 2PbO (s)
1. Lead (Pb) is in the solid state
2. 2 moles of product are formed with 2 moles of lead reacts with 1 mole of oxygen
3. Lead is a product
15
A scientist sets up a reaction with 65.4 g of dimethyl ether (C2H6O) to react with excess oxygen, how many grams of water will be formed?
__C2H6O+__O2-->__CO2+__H2O
D
Which of the following would be the wavelength with the highest energy?
A) 300 km
B) 300 cm
C) 300 m
D) 300 nm
E) 300 mm
9.19 x 10^3 micro m
Using the information below how many micrometers are in 0.362 inches?
1 inch = 2.54 cm
1 micro m = 1x10^-6 m
10 core electrons, 6 valence electrons
How many core electrons and valance electrons does sulfur (S) have?
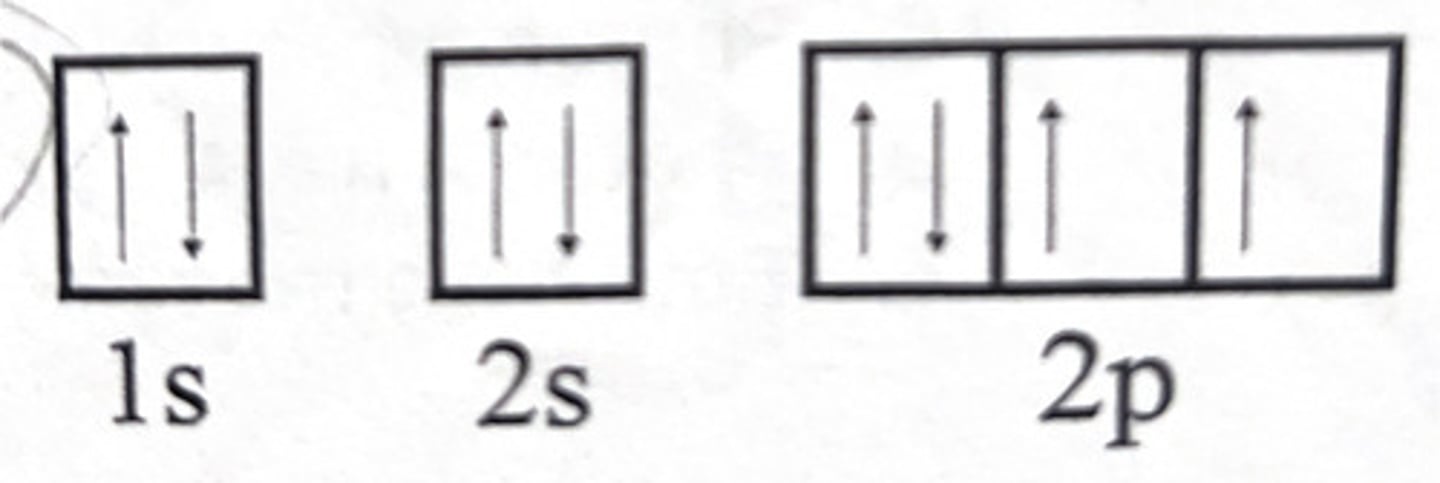
Draw the orbital diagram that represents an oxygen atom (O)?
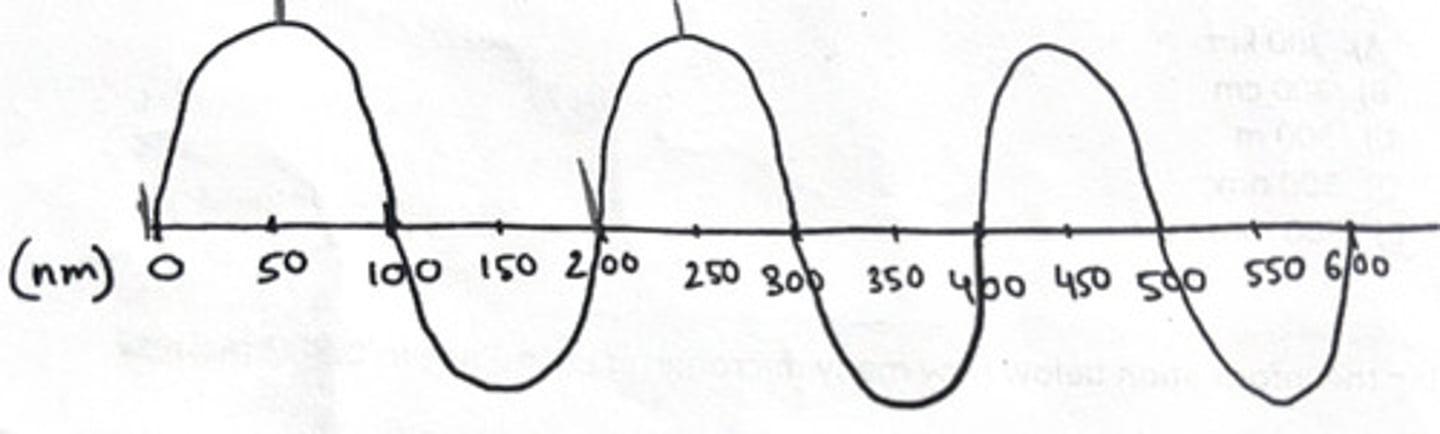
200 nm
What is the wavelength of the wave drawn below?
II
If the intensity of a beam of light is decreased, this would correspond to:
I. An increase in the number of photons (considering light as a particle).
II. A decrease in the amplitude of the wave (considering light as a wave).
III. An increase in the wavelength of the light.
A
Photon A has a wavelength of 565 nm and Photon B has a wavelength of 430 nm. From the options below, select the true statement.
A) Photon A has a lower frequency and lower energy than Photon B.
B) Photon A has a lower frequency and higher energy than Photon B.
C) Photon A has a higher frequency and higher energy than Photon B.
D) Photon A has a higher frequency and lower energy than Photon B.
E) Both have the same frequency and the same energy.
75.3%
If 25.5 g of acetone (C3H6O) reacts with excess oxygen, what is the percent yield if 43.7 g of CO2 is formed via the equation below?
C3H6O + 4O2 --> 3CO2 + 3H2O
97.8 g
In the wine-making industry, yeast is used to convert sugar (glucose, C6H12O6) from the squashed grapes into ethanol (C5H5OH) and carbon dioxide (CO2). The balanced chemical equation is provided below.
C6H12O6 --> 2C2H5OH + 2CO2
What mass of sugar (C6H12O6) would be needed to produce 50.0 g of ethanol (C6H12O6)?
19.7 moles
How many moles of water (H2O) are present in 355g of water?
1, 2, and 3
Which of the following about absorption and emission spectra is/are false?
1. Absorption spectra show bright lines on a dark background, indicating wavelengths of light absorbed by electrons.
2. Emission spectra show dark lines on a continuous spectrum, representing specific wavelengths of light that are emitted by electrons.
3. All elements produce identical absorption spectra, but different emission spectra.
4. Absorption spectra arise when electrons in atoms absorb energy and transition to a higher energy level.
5. Emission spectra arise when electrons relax to a lower energy level and emit energy.
2 and 4
Given electron configuration of an element to be 1s2 2s2 2p6 3s2 3p3, which of the following statements are true?
1. The element is sulfur.
2. The element is phosphorus.
3. It has 4 valence electrons.
4. It has 10 core electrons.
[Ar] 4s2 3d10 4p2
What is the correct electron configuration of Germanium (Ge)?
2 and 5
Which substance has the lower boiling point and why?
1. Br2
2. Cl2
Because
3. Br2 has weaker bonds than Cl2 and therefore takes less energy to break the bond.
4. Cl2 has weaker bonds than Br2 and therefore takes less energy to break the bond when Cl2 boils.
5. Cl2 has weaker LDFs than Br2 and therefore takes less energy to overcome the LDFs when Cl2 boils.
6 Br2 has weaker LDFs than Cl2 and therefore takes less energy to overcome the LDFs when Br2 boils.
1 and 5
Which of the following has the highest ionization energy?
1. Br
2. Ge
3. Se
Because
4. It has a lower ionization energy because there are more core electrons.
5. It has a higher ionization energy because there are more protons but no additional core electrons.
6. It has a higher ionization energy because there are less valence electrons.
3 and 4
The ions S2- , K+ and Ca2+ are all isoelectronic with the noble gas Ar, which is the smallest ion and why?
1. S2-
2. K+
3. Ca2+
Because
4. Because all the ions have the same number of electrons, but the smallest ion has more protons.
5. Because ions on the right side of the periodic table are smaller than those on the left side of the periodic table.
6. Because ions on the left side of the periodic table are smaller than those on the right side of the periodic table.
Metallic bonding
A substance has the following properties
Color: silvery-white
Melting point: 1828 K
Conducts electricity
Malleable
What type of bonding/interactions does the substance have that gave the substance its properties?
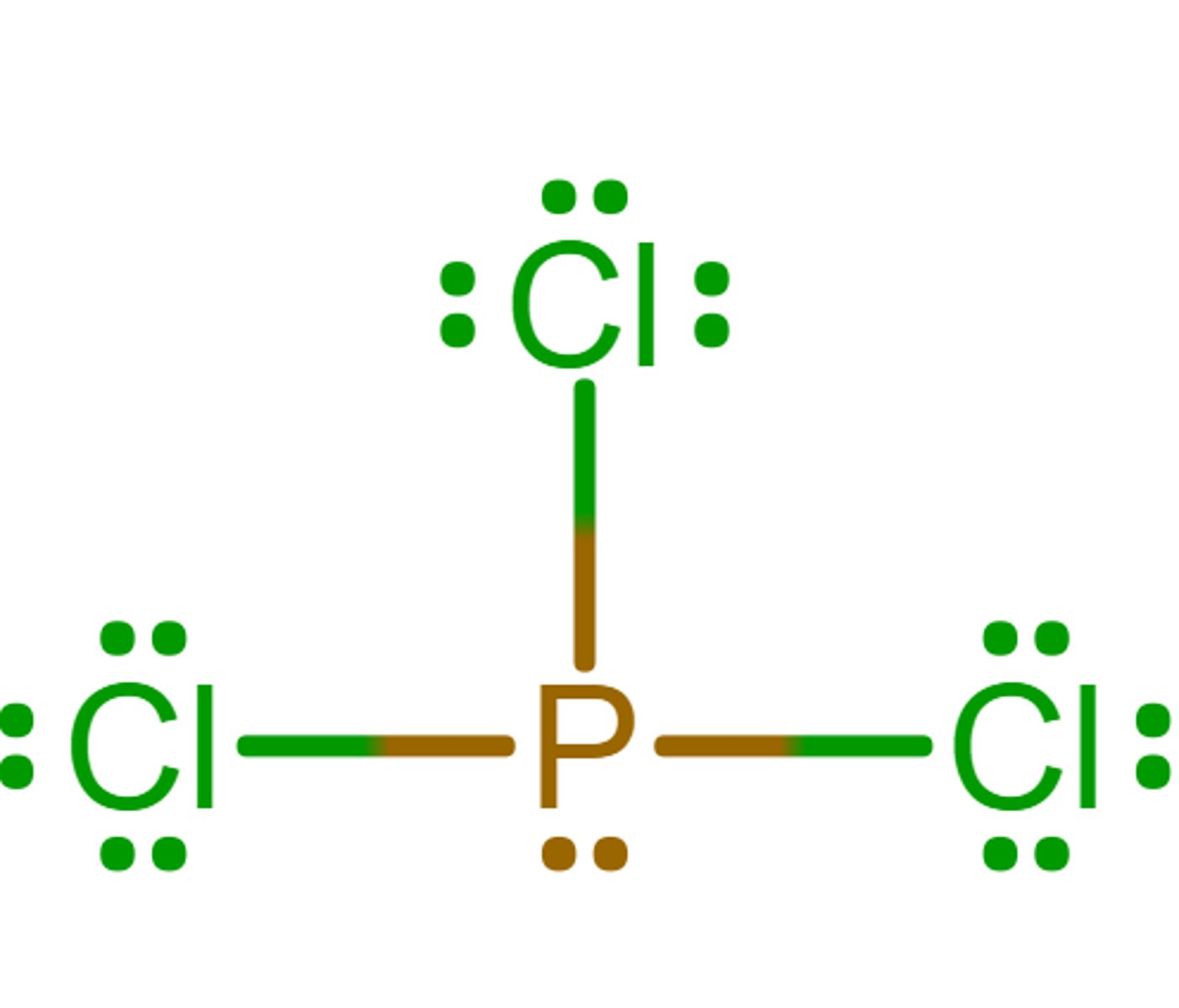
Tetrahedral
What is the electron geometry?
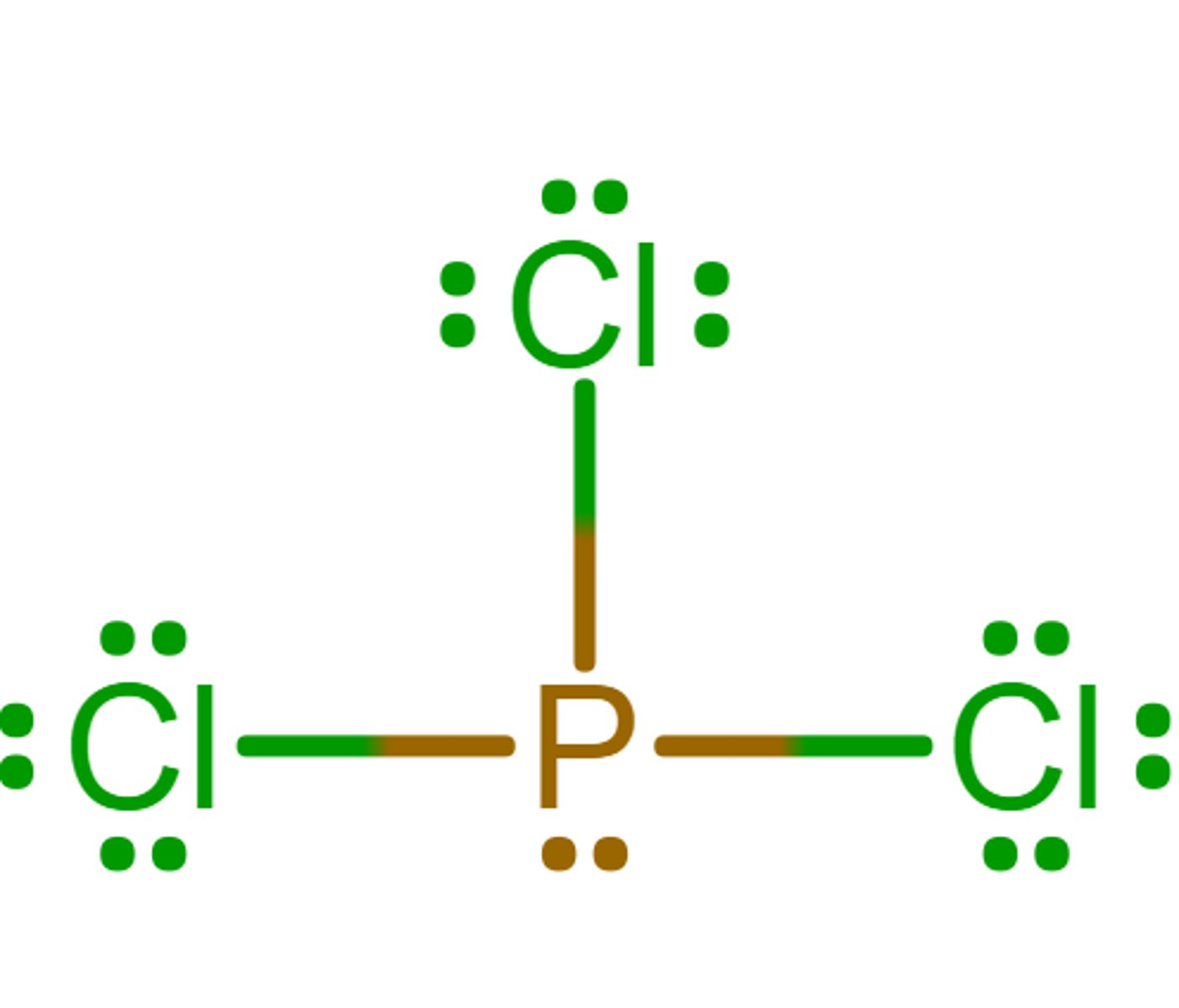
Trigonal pyramidal
What is the molecular geometry/molecular shape?
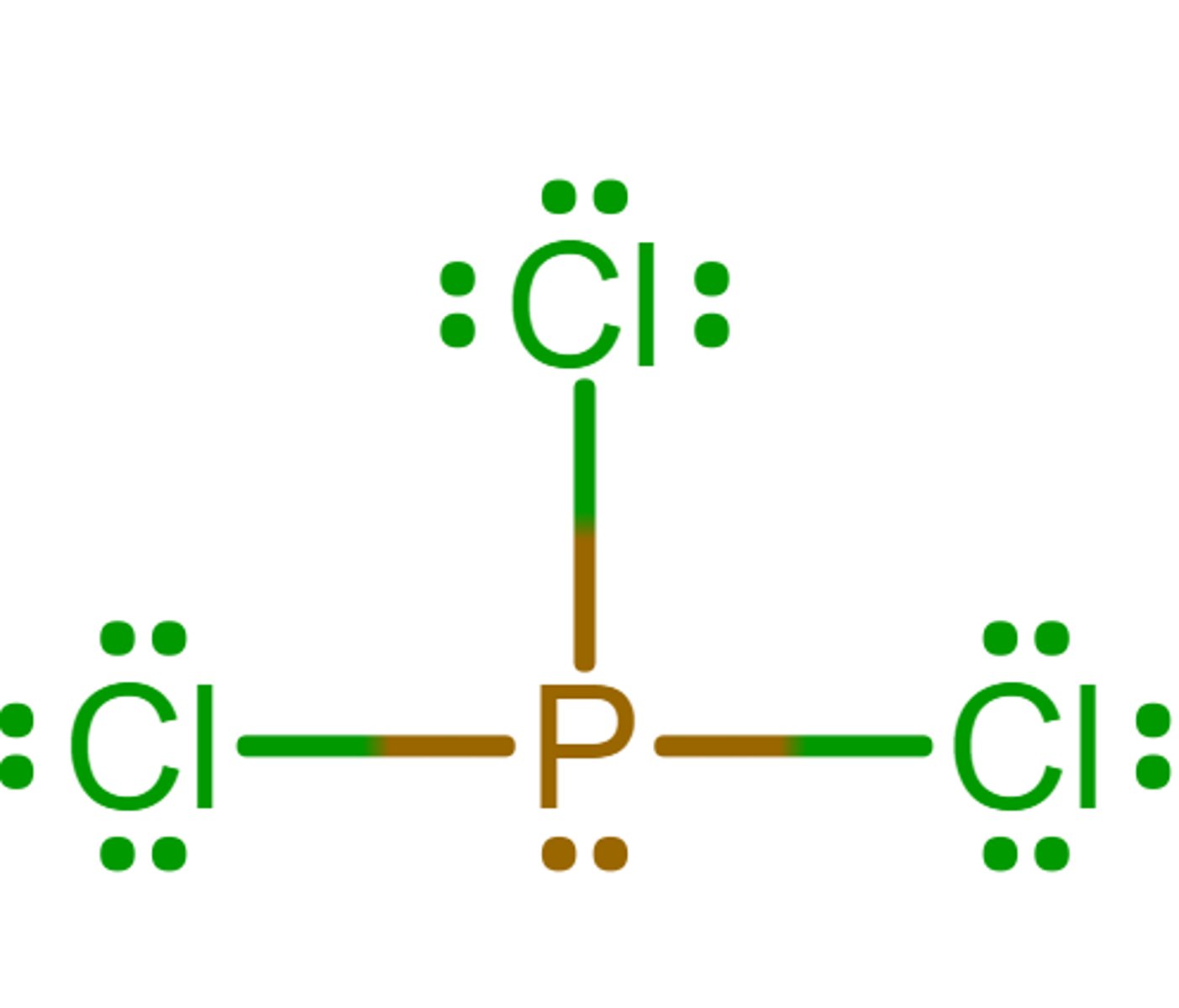
sp3
What is the hybridization?
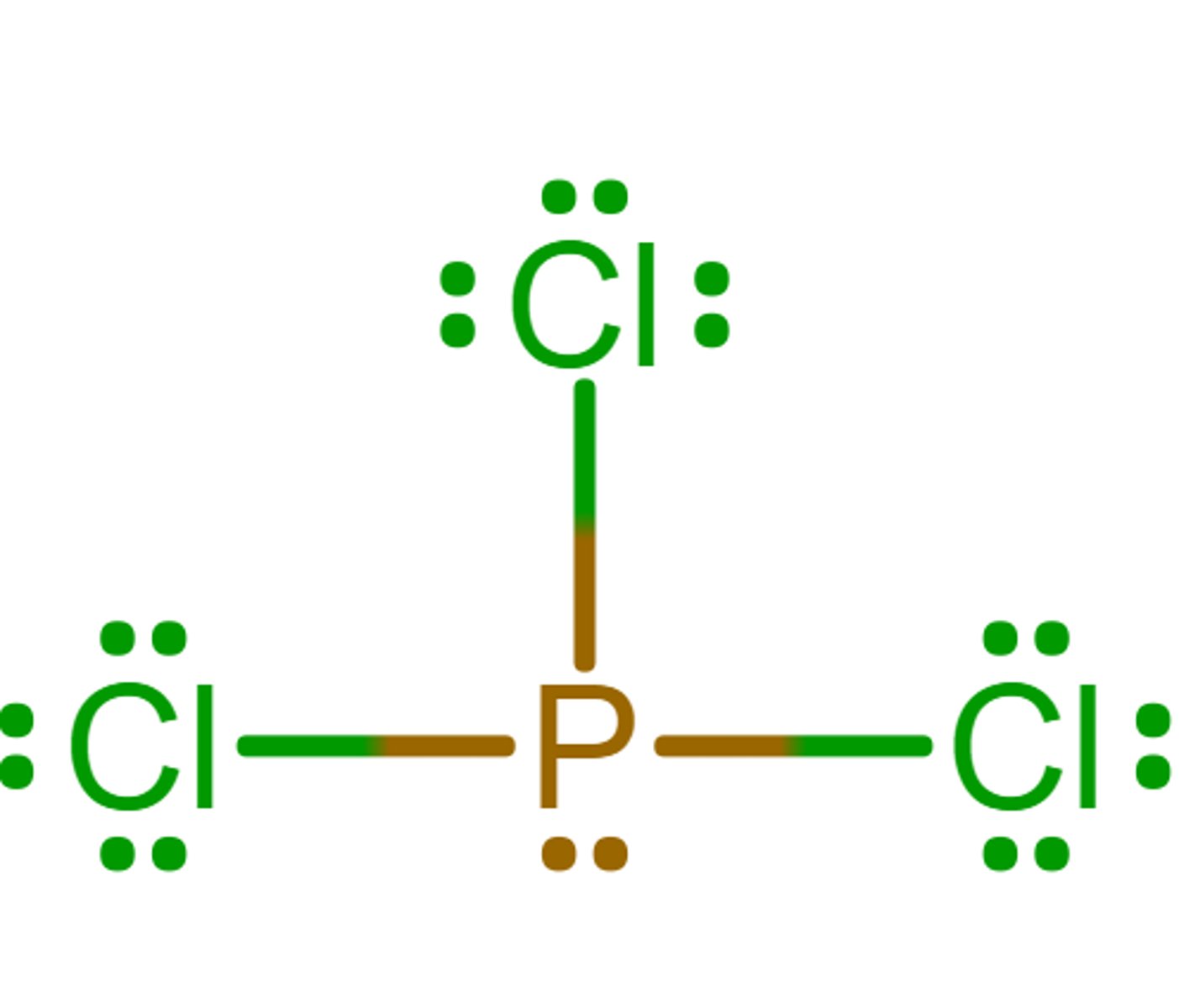
0
What is the formal charge of phosphorus in the molecule?
1 and 3
Which of the following bonds does not rotate and why?
1. The double bond between two carbons
2. The single bond between two carbons
Because
3. This bond consists of a pi bond formed by the orbitals overlapping side to side.
4. This bond is a sigma bond formed by the orbitals overlapping end to end.
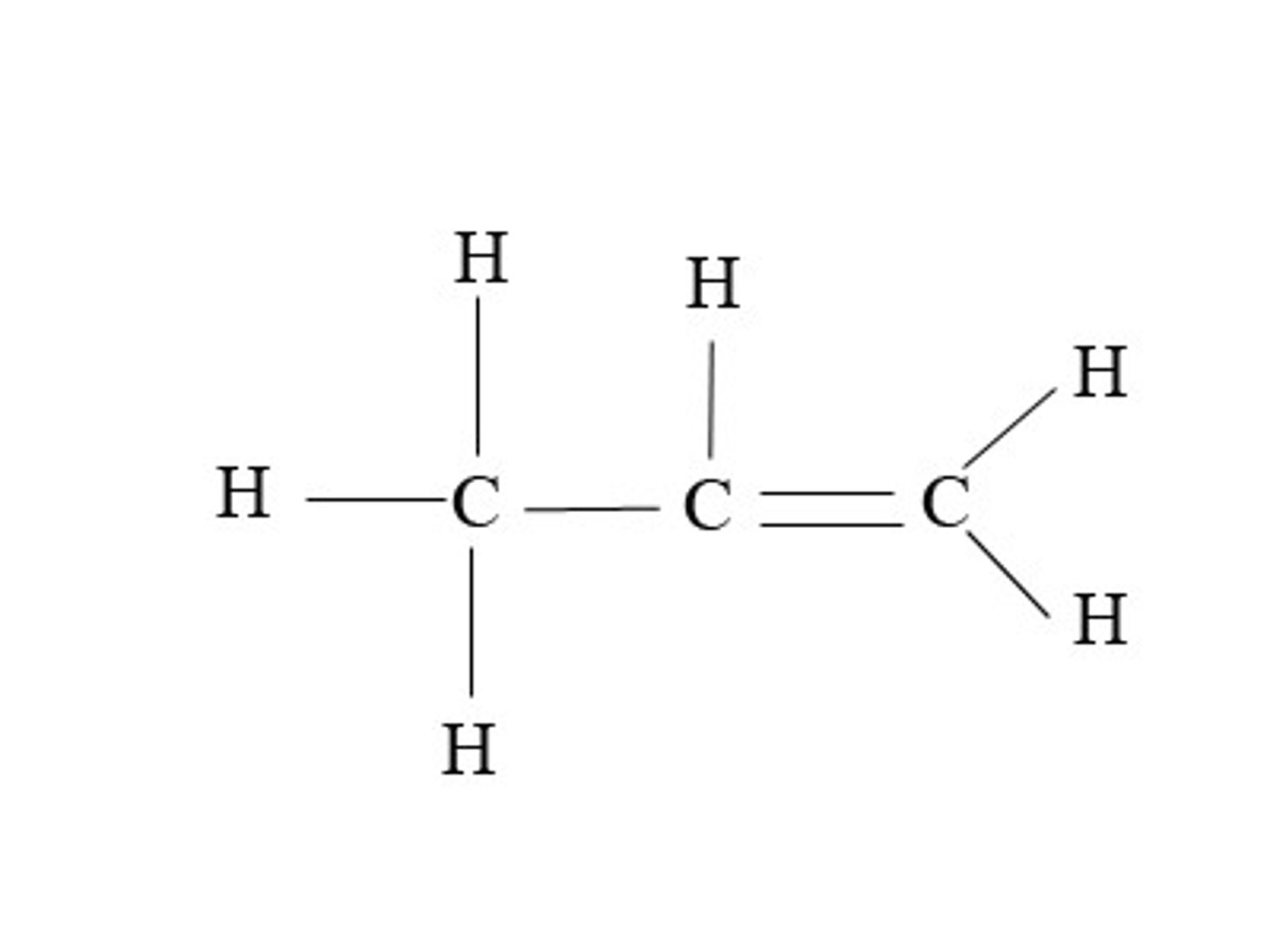
2 and 4
Why do covalent bonds form?
1) Because atoms want to share electrons.
2) Because electrons from one atom are attracted to the nucleus of another atom.
3) Because atoms want an octet of electrons around them.
4) Because the formation of a bond results in a stable system that would require the input of energy to change.
II only
Which of the following Lewis structures could be correct representation(s) for the molecule with the chemical formula CH5N?
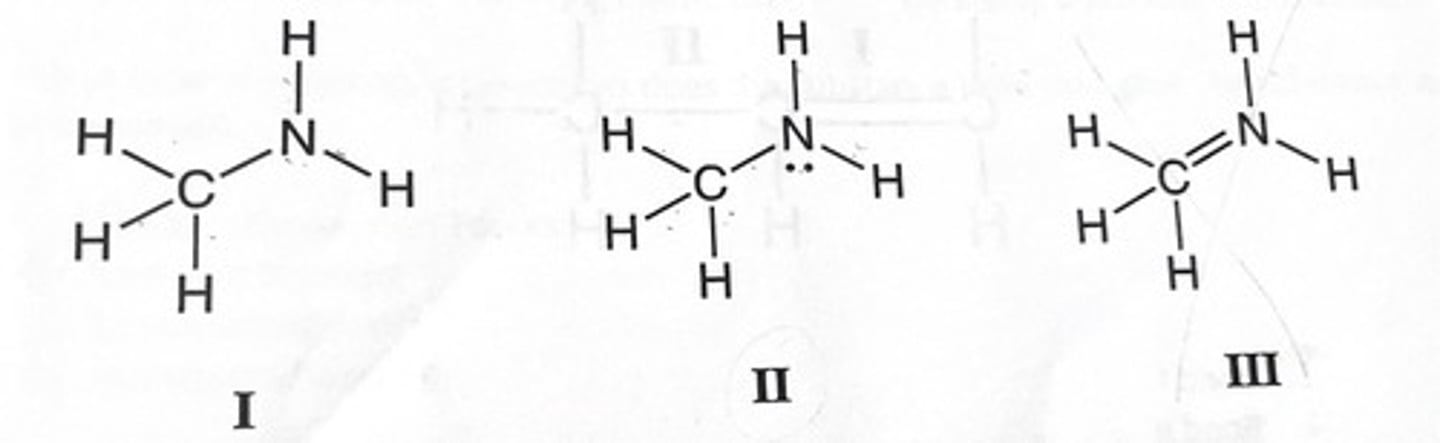
D
Do the following structures represent the same molecule or different molecules?
A) Same molecule, because they have the same chemical formula.
B) Different molecules, because they have different numbers of carbon and hydrogen atoms.
C) Same molecule, because they have the same atoms, but the representations are different.
D) Different molecules because the connectivity is different.
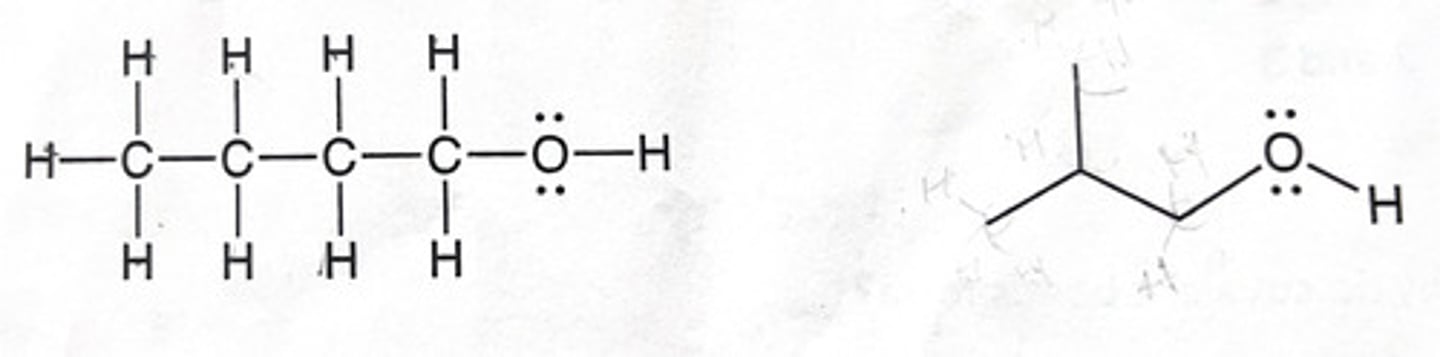
II
Which of the following molecule(s) is polar?
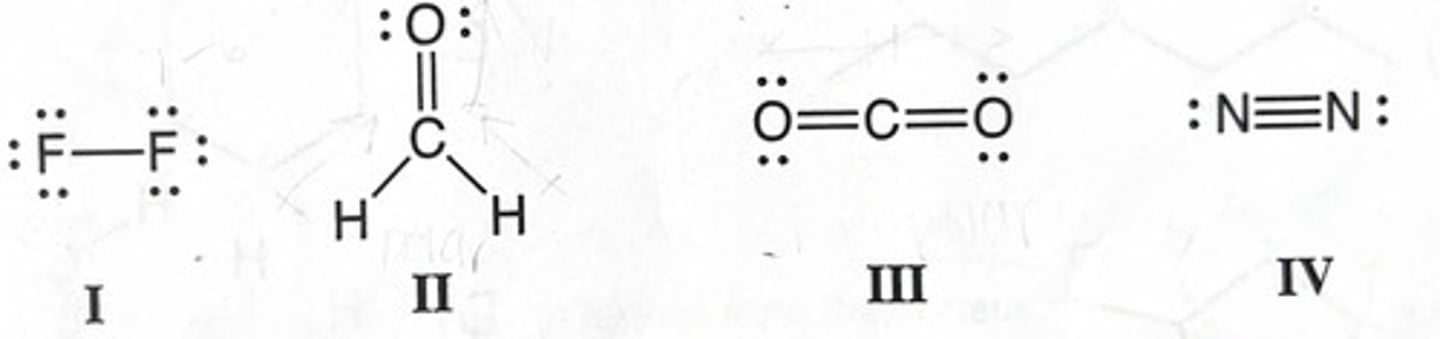
+1
A Lewis structure is shown for the nitrate ion (NO3-). What is the formal charge on nitrogen in the nitrate ion?
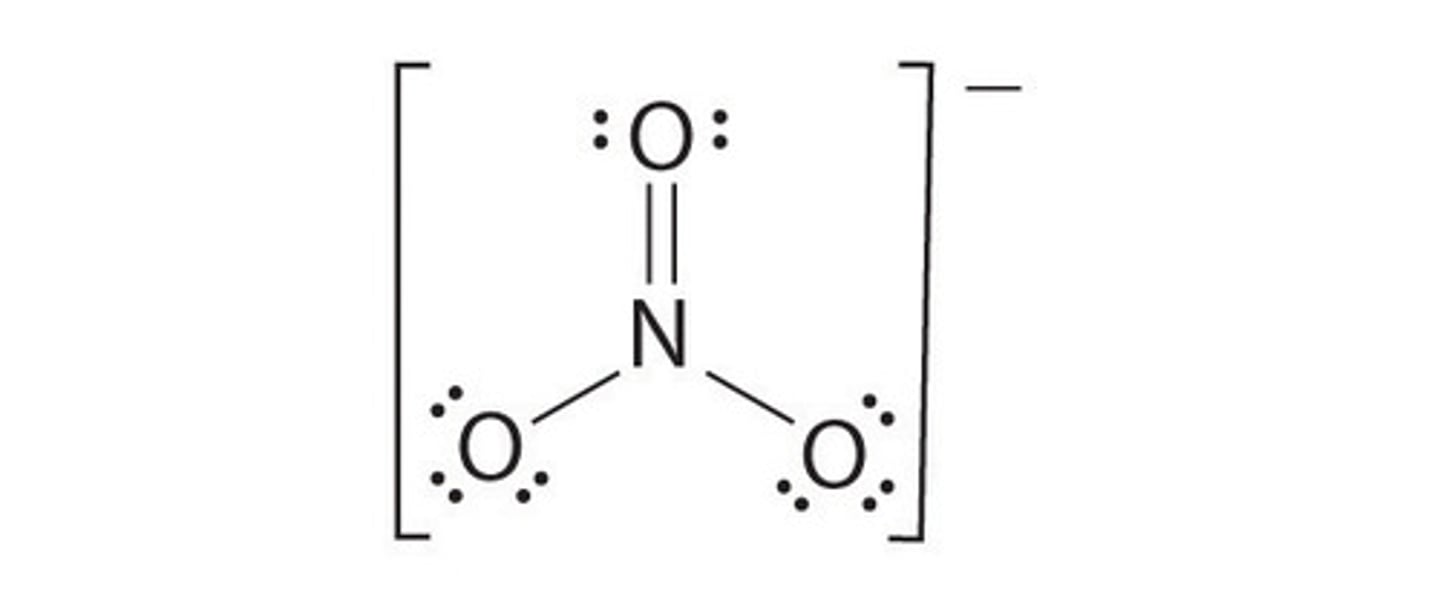
2 and 4
The potential energy curve, shown below, is for two nuclei undergoing nuclear fusion. As the nuclei are very close to each other, the potential energy decreases. Why does it decrease?
1) The dominant force between the nuclei is repulsive.
2) The dominant force between the nuclei is attractive.
Because
3) Nuclei are positively charged and interact through the electrostatic force.
4) The strong nuclear force acts on nuclei over very short distances.
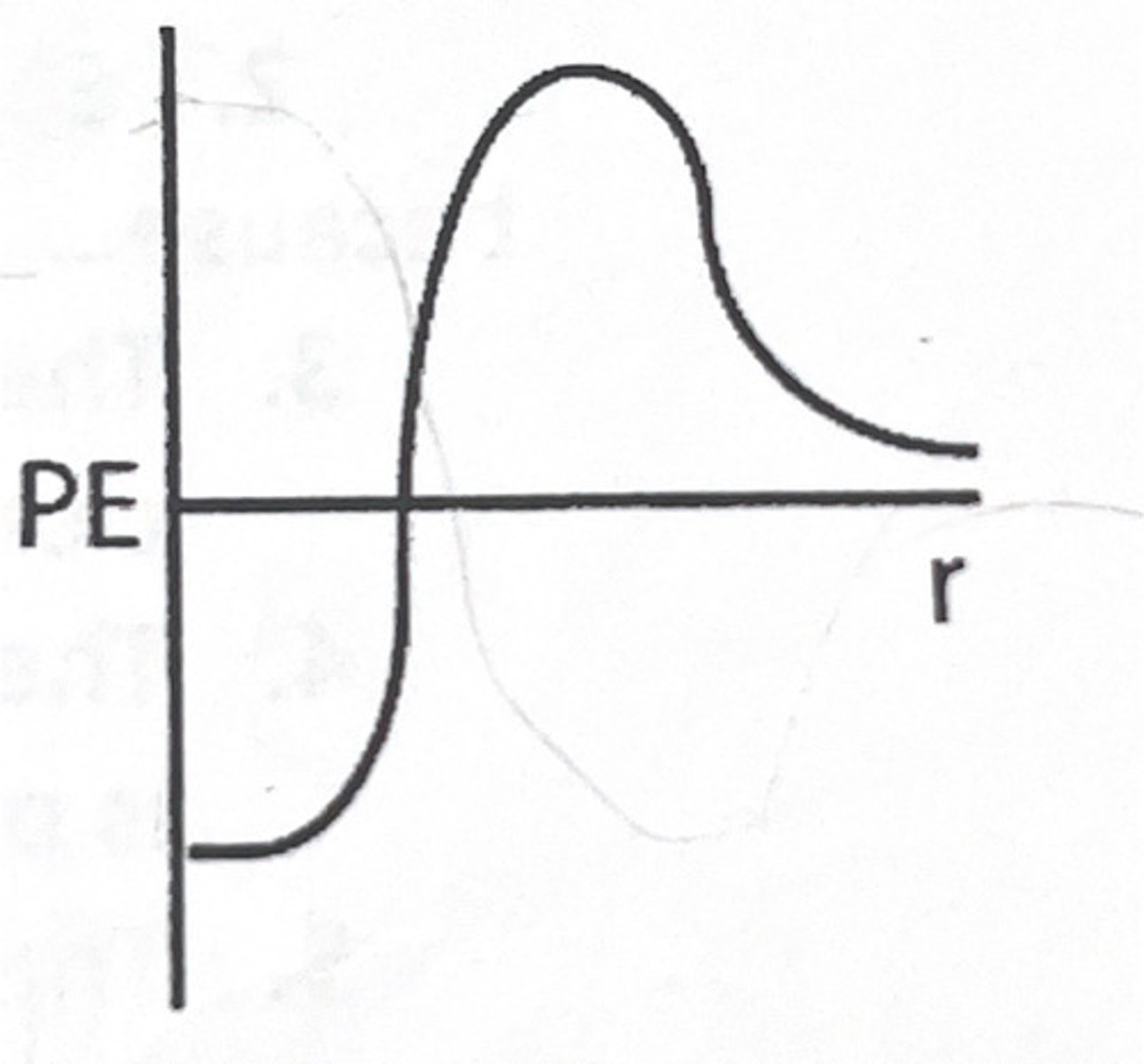
A
Which of the following is not a valid representation of C6H12?
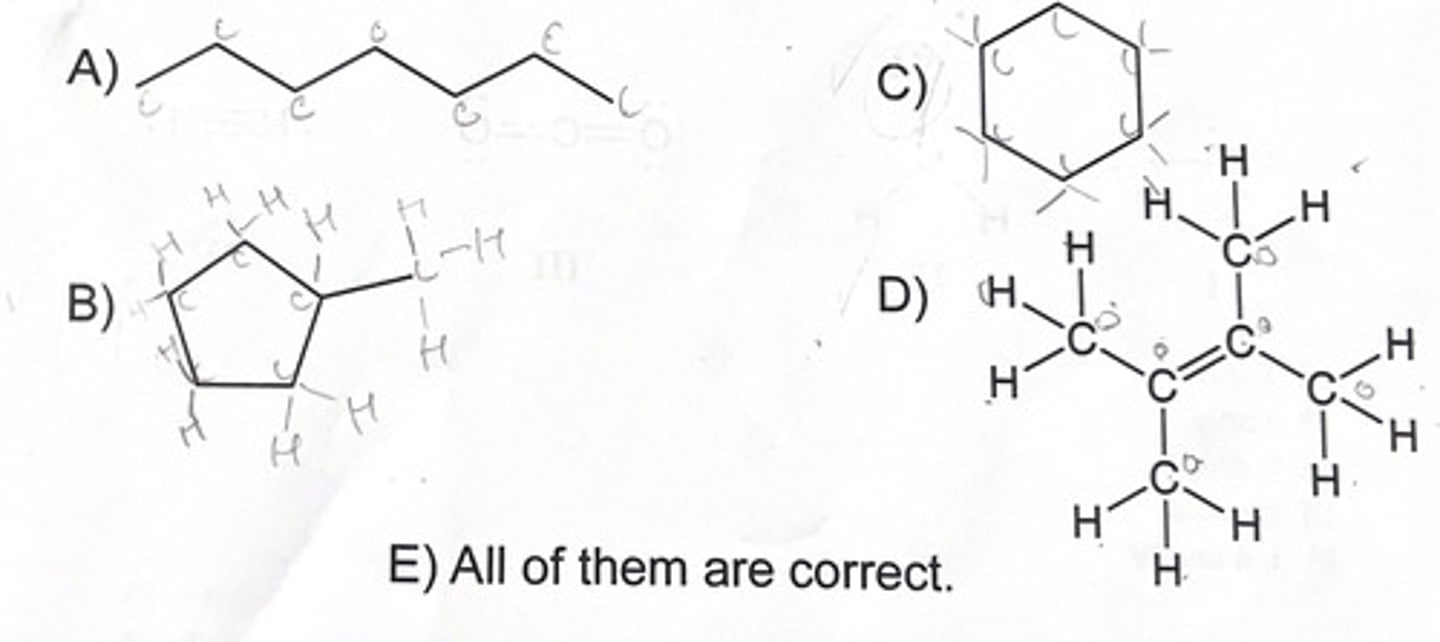
2 and 4
Consider imaginary element E. Which would you expect to have the largest radius and why?
1. E+
2. E-
because
3. There are fewer electron-electron repulsions in this species, so the electron cloud is pushed out farther from the nucleus.
4. There are more electron-electron repulsions in this species, so the electron cloud is pushed out farther from the nucleus.
5. There is a higher effective nuclear charge, so the valance shell is pushed farther away from the nucleus.
2 and 4
Which element is less electronegative?
1. S
2. P
because
3. the valance electrons are further from the nucleus.
4. the valance electrons experience a lower effective nuclear charge.
5. sulfur has more total electrons than phosphorus.
D
Which has a larger atomic radius? Be or O and why.
A) Be, because it is a metal.
B) O, because it is a gas.
C) O, because it has a higher effective nuclear charge meaning its valence electrons are more strongly attracted towards the nucleus.
D) Be, because it has a lower effective nuclear charge meaning its valence electrons are not as strongly attracted towards the nucleus.
E) O, because it has more electrons, and they repel each other.
A
Which of the following element(s) does not conduct electricity?
A) Diamond (C)
B) Copper (Cu)
C) Lithium (Li)
D) Graphite (C)