Biosci 221 Lab Quiz 6
5.0(1)
Card Sorting
1/51
Earn XP
Description and Tags
Dilution Problem, Physiological tests, Staining, Protists, Fungi, and Helminths
Last updated 9:50 PM on 5/16/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
1
New cards
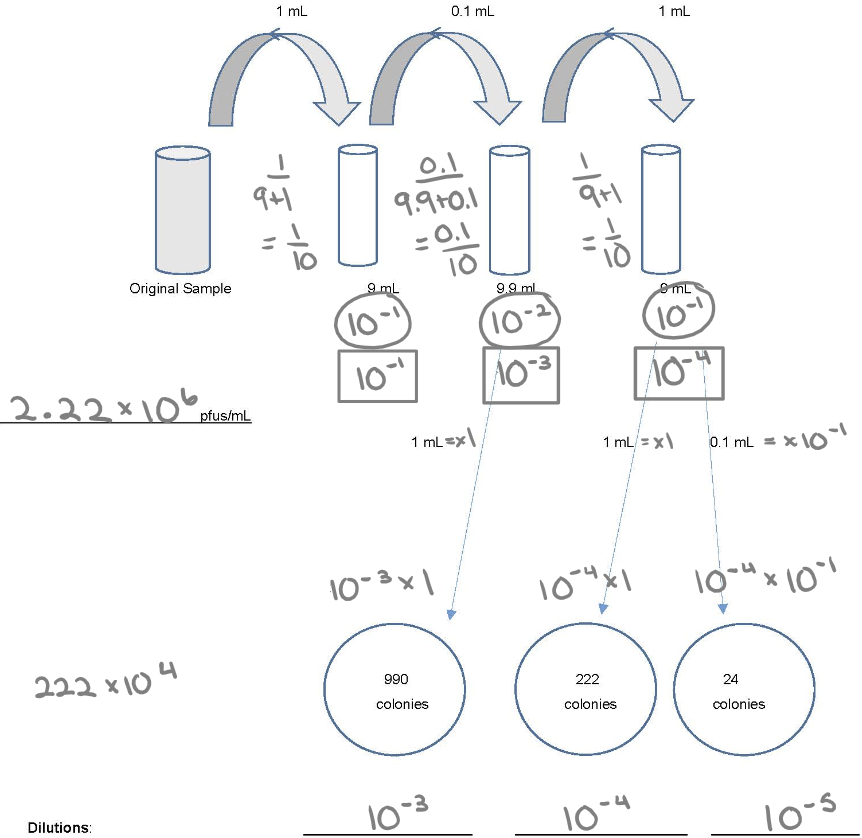
Know how to do dilution
1. Individual dilution
2. Total dilution
3. How much plating: 1.0 ml (x1) or 0.1 ml (x 10^-1)
4. (30-300) x DF
2
New cards
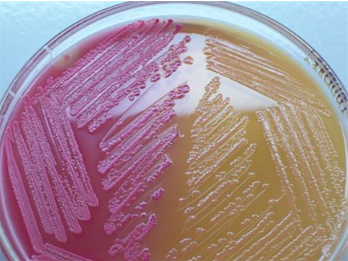
MacConkey agar (MAC)
* **Purpose**: to differentiate between lactose fermenting G- bacteria and lactose non-fermenting G- bacteria
* **Medium**: crystal violet and bile salts
* **Type of Medium**: selective and differential
* **+ results** = pink colonies
* **- results** = no color change
* **significant specific ingredients**:
* **reagents/indicators**:
* Crystal violet and bile salts inhibit Gram (+) bacteria
* and neutral red dye stains microbes fermenting lactose (and thereby decreasing the pH) a pink color.
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results**:
* **Medium**: crystal violet and bile salts
* **Type of Medium**: selective and differential
* **+ results** = pink colonies
* **- results** = no color change
* **significant specific ingredients**:
* **reagents/indicators**:
* Crystal violet and bile salts inhibit Gram (+) bacteria
* and neutral red dye stains microbes fermenting lactose (and thereby decreasing the pH) a pink color.
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results**:
3
New cards
Columbia Nalidixic Acid agar (CNA)
* **Purpose**: for the isolation of G+ cocci
* **Medium**: contains the antibiotics colistin and nalidixic acid
* **Type of Medium**: selective and differential
* Growth (G) = clearly visible, good growth
* Weak growth (WG) = very little growth
* No growth (NG) = no visible growth
* **significant specific ingredients**:
* **reagents/indicators**: colistin and nalidixic acid inhibit G- bacteria
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results:**
* **Medium**: contains the antibiotics colistin and nalidixic acid
* **Type of Medium**: selective and differential
* Growth (G) = clearly visible, good growth
* Weak growth (WG) = very little growth
* No growth (NG) = no visible growth
* **significant specific ingredients**:
* **reagents/indicators**: colistin and nalidixic acid inhibit G- bacteria
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results:**
4
New cards
Catalase
* **Purpose**: to detect the production of the enzyme, catalase
* **Medium**: TSA
* **Type of Medium**: differential
* **+ results** = bubbling due to the release of oxygen via catalase
* **- results** = no bubbling
* **significant specific ingredients:**
* **reagents/indicators**: 3% Hydrogen Peroxide (H2O2)
* **specific directions** (if any): apply 3% hydrogen peroxide to growth from a TSA plate
* **significant results:**
* **Medium**: TSA
* **Type of Medium**: differential
* **+ results** = bubbling due to the release of oxygen via catalase
* **- results** = no bubbling
* **significant specific ingredients:**
* **reagents/indicators**: 3% Hydrogen Peroxide (H2O2)
* **specific directions** (if any): apply 3% hydrogen peroxide to growth from a TSA plate
* **significant results:**

5
New cards
Oxidase
* **Purpose**: to detect the production of the enzyme cytochrome c oxidase
* **Medium**: use growth from a TSA plate or slant
* **Type of Medium:** differential
* **+ results** = color change to purple within about 30 sec
* **- results** = no color change or a change after more than 30 sec
* **significant specific ingredients:**
* **reagents/indicators:** oxidase dry slides; used after growth on TSA.
* **specific directions** (if any): using a sterile wooden stick, pick a colony of bacteria from a TSA plate or slant and touch an area on one section of the dry slide
* **significant results:**
* **Medium**: use growth from a TSA plate or slant
* **Type of Medium:** differential
* **+ results** = color change to purple within about 30 sec
* **- results** = no color change or a change after more than 30 sec
* **significant specific ingredients:**
* **reagents/indicators:** oxidase dry slides; used after growth on TSA.
* **specific directions** (if any): using a sterile wooden stick, pick a colony of bacteria from a TSA plate or slant and touch an area on one section of the dry slide
* **significant results:**

6
New cards
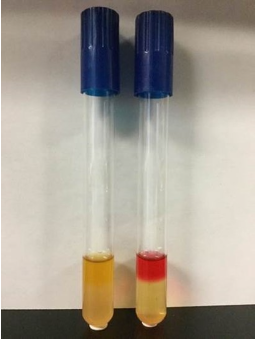
Methyl Red test (MR)
* **Purpose**: to determine mixed acid fermentation (lactic, acetic, formic, etc)
* Part of the IMViC tests.
* **Medium**: MRVP broth--buffered peptone glucose broth
* **Type of Medium:** differential
* **+ results** = red
* **- results** = yellow
* **weak +** = orange
* **significant specific ingredients:**
* **reagents/indicators**: methyl red (added AFTER incubation)
* **specific directions** (if any): broth is inoculated and incubated. After incubation, add 5 drops of Methyl Red indicator, do not shake the tube, and read the results immediately
* **significant results:**
* Part of the IMViC tests.
* **Medium**: MRVP broth--buffered peptone glucose broth
* **Type of Medium:** differential
* **+ results** = red
* **- results** = yellow
* **weak +** = orange
* **significant specific ingredients:**
* **reagents/indicators**: methyl red (added AFTER incubation)
* **specific directions** (if any): broth is inoculated and incubated. After incubation, add 5 drops of Methyl Red indicator, do not shake the tube, and read the results immediately
* **significant results:**
7
New cards
Voges Proskauer test (VP)
* **Purpose**: to detect the production of acetoin or butanediol from the fermentation of glucose in the broth
* Part of the IMViC tests.
* **Medium**: MRVP broth-- buffered glucose peptone broth
* **Type of Medium**: differential
* **+ results** = red layer at the top in 10 minutes (earliest detection), progressing downward
* **- results** = no red color, disregard any copper or brownish-purple color
* **significant specific ingredients:**
* **reagents/indicators:** Barritt’s reagents A and B added AFTER incubation
* **specific directions** (if any): inoculate broth and incubate. After incubation add 20 drops of Barritt’s Reagent A and 20 drops of Barritt’s Reagent B. Vortex at frequent intervals and allow the reaction to develop for up to 1 – 2 hours.
* **significant results:**
* Part of the IMViC tests.
* **Medium**: MRVP broth-- buffered glucose peptone broth
* **Type of Medium**: differential
* **+ results** = red layer at the top in 10 minutes (earliest detection), progressing downward
* **- results** = no red color, disregard any copper or brownish-purple color
* **significant specific ingredients:**
* **reagents/indicators:** Barritt’s reagents A and B added AFTER incubation
* **specific directions** (if any): inoculate broth and incubate. After incubation add 20 drops of Barritt’s Reagent A and 20 drops of Barritt’s Reagent B. Vortex at frequent intervals and allow the reaction to develop for up to 1 – 2 hours.
* **significant results:**

8
New cards
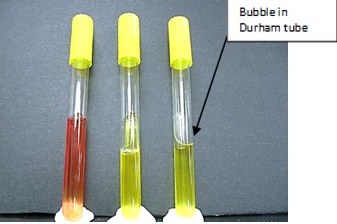
Phenol Red Broths - Lactose, Dextrose (Glucose), Sucrose
* **Purpose**: to distinguish carbohydrate fermenters from non-fermenters & to detect and distinguish the different, specific carbohydrates by the products formed
* **Medium**: 0.5% to 1% carbohydrate (dextrose/lactose/sucrose) broth, peptone, with phenol red and an inverted Durham tube for detection of gas.
* **Type of Medium:** general purpose differential test media
* **+ results** = turns from red to yellow
* **- results** = no color change
* **Gas production (+)** = bubble trapped in an inverted Durham tube
* **No gas production (-)** = no bubble trapped in an inverted Durham tube
* **significant specific ingredients:**
* **reagents/indicators:** phenol red as a pH indicator to indicate acid production
* **specific directions** (if any): inoculate tubes and incubate
* **significant results:**
* AG = acid with gas production
* A = acid, no gas
* (-) = negative for acid and gas
* **Medium**: 0.5% to 1% carbohydrate (dextrose/lactose/sucrose) broth, peptone, with phenol red and an inverted Durham tube for detection of gas.
* **Type of Medium:** general purpose differential test media
* **+ results** = turns from red to yellow
* **- results** = no color change
* **Gas production (+)** = bubble trapped in an inverted Durham tube
* **No gas production (-)** = no bubble trapped in an inverted Durham tube
* **significant specific ingredients:**
* **reagents/indicators:** phenol red as a pH indicator to indicate acid production
* **specific directions** (if any): inoculate tubes and incubate
* **significant results:**
* AG = acid with gas production
* A = acid, no gas
* (-) = negative for acid and gas
9
New cards
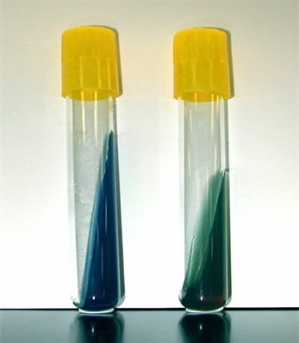
Simmon's Citrate test
* **Purpose**: to determine an organism’s ability to use citrate as the sole source of carbon
* Part of the IMViC tests.
* **Medium**: Simmons Citrate Agar- contains sodium citrate as the sole carbon source, mineral salts, and pH indicator Bromothymol blue
* **Type of Medium:** selective and differential
* **+ results** = color change from green to blue
* **- results** = no color change
* **significant specific ingredients:**
* **reagents/indicators:** Bromothymol blue is a pH indicator
* **specific directions** (if any): streak slant, cap loosely, and incubate.
* **significant results:**
* Part of the IMViC tests.
* **Medium**: Simmons Citrate Agar- contains sodium citrate as the sole carbon source, mineral salts, and pH indicator Bromothymol blue
* **Type of Medium:** selective and differential
* **+ results** = color change from green to blue
* **- results** = no color change
* **significant specific ingredients:**
* **reagents/indicators:** Bromothymol blue is a pH indicator
* **specific directions** (if any): streak slant, cap loosely, and incubate.
* **significant results:**
10
New cards
Starch hydrolysis
* **Purpose**: to detect the production of the enzyme amylase
* **Medium**: Starch Agar plates (1% starch)
* **Type of Medium:** differential
* **+ results** = clear zone around growth
* **- results** = no zone
* **significant specific ingredients:**
* **reagents/indicators:** Gram’s Iodine
* **specific directions** (if any): streak agar in a straight line and incubate. After incubation, add Gram’s iodine, dropwise, sparingly, just to cover growth and surrounding area on the medium. Let the plate sit for a few minutes for the reaction to develop
* **significant results:**
* **Medium**: Starch Agar plates (1% starch)
* **Type of Medium:** differential
* **+ results** = clear zone around growth
* **- results** = no zone
* **significant specific ingredients:**
* **reagents/indicators:** Gram’s Iodine
* **specific directions** (if any): streak agar in a straight line and incubate. After incubation, add Gram’s iodine, dropwise, sparingly, just to cover growth and surrounding area on the medium. Let the plate sit for a few minutes for the reaction to develop
* **significant results:**

11
New cards
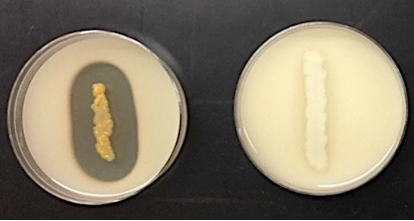
Casein hydrolysis (Skim Milk)
* **Purpose**: to detect the production of the enzyme casease
* **Medium**: Skim Milk Agar
* **Type of Medium:** differential
* **+ results** = clear zone around growth
* **- results** = no clear zone
* **significant specific ingredients:**
* **reagents/indicators:**
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results:**
* **Medium**: Skim Milk Agar
* **Type of Medium:** differential
* **+ results** = clear zone around growth
* **- results** = no clear zone
* **significant specific ingredients:**
* **reagents/indicators:**
* **specific directions** (if any): streak agar in a straight line and incubate
* **significant results:**
12
New cards
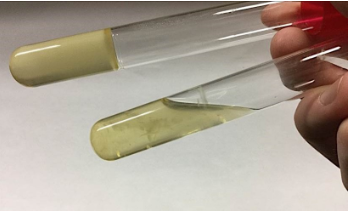
Urease production
* **Purpose**: to detect the production of the enzyme urease
* **Medium**: urea broth
* **Type of Medium:** differential
* **+ results** = red or bright pink color
* **- results** = yellow color
* **significant specific ingredients:**
* **reagents/indicators:** Phenol Red
* **specific directions (if any)**: inoculate urea broth and incubate
* **significant results:**
* **Medium**: urea broth
* **Type of Medium:** differential
* **+ results** = red or bright pink color
* **- results** = yellow color
* **significant specific ingredients:**
* **reagents/indicators:** Phenol Red
* **specific directions (if any)**: inoculate urea broth and incubate
* **significant results:**

13
New cards
Gelatin Liquefaction
* **Purpose**: to determine the production of gelatinase
* **Medium**: Nutrient Gelatin Deep (12- 15% gelatin)
* **Type of Medium:** differential
* **+ results** = Liquefaction (after refrigeration)
* **- results** = Gels when refrigerated, no liquefaction
* **significant specific ingredients:**
* **reagents/indicators:**
* **specific directions** (if any): deep stab inoculation and incubate. After incubation, refrigerate for 1 hour before reading.
* **significant results:**
* **Medium**: Nutrient Gelatin Deep (12- 15% gelatin)
* **Type of Medium:** differential
* **+ results** = Liquefaction (after refrigeration)
* **- results** = Gels when refrigerated, no liquefaction
* **significant specific ingredients:**
* **reagents/indicators:**
* **specific directions** (if any): deep stab inoculation and incubate. After incubation, refrigerate for 1 hour before reading.
* **significant results:**
14
New cards
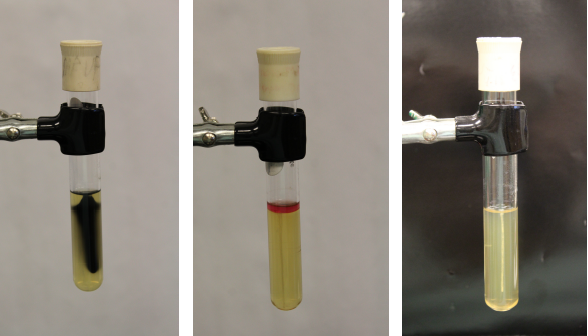
Sulfide, Indole, Motility (SIM) Medium
* **Purpose**: to differentiate G- rods
* **Medium**: contains casein peptone, ferrous ammonium sulfate, sodium thiosulfate, and agar
* **Type of Medium**: selective and differential
* **+ results:**
* Sulfide (+) = blackening of the media
* Indole (+) = pink to red color observed on the surface after the addition of Kovac’s reagent
* Motility (+) = growth observed beyond the stab line (cloudy, turbid)
* **- results:**
* Sulfide (-) = no blackening of the media observed
* Indole (-) = yellow color observed on the surface after addition of Kovac’s reagent
* Motility (-) = no growth observed beyond stab line (crisp solid inoculation line visible)
* **significant specific ingredients:**
* **reagents/indicators:**
* ferrous ammonium sulfate and sodium thiosulfate are present to detect hydrogen sulfide (H2S) production
* The agar in the medium creates a semi-solid environment for motility to be visualized
* Kovac’s reagent (added AFTER incubation) will detect indole production
* **specific directions** (if any): inoculate the media using your needle, stabbing down the center of the media in the tube
* **significant results:**
* **Medium**: contains casein peptone, ferrous ammonium sulfate, sodium thiosulfate, and agar
* **Type of Medium**: selective and differential
* **+ results:**
* Sulfide (+) = blackening of the media
* Indole (+) = pink to red color observed on the surface after the addition of Kovac’s reagent
* Motility (+) = growth observed beyond the stab line (cloudy, turbid)
* **- results:**
* Sulfide (-) = no blackening of the media observed
* Indole (-) = yellow color observed on the surface after addition of Kovac’s reagent
* Motility (-) = no growth observed beyond stab line (crisp solid inoculation line visible)
* **significant specific ingredients:**
* **reagents/indicators:**
* ferrous ammonium sulfate and sodium thiosulfate are present to detect hydrogen sulfide (H2S) production
* The agar in the medium creates a semi-solid environment for motility to be visualized
* Kovac’s reagent (added AFTER incubation) will detect indole production
* **specific directions** (if any): inoculate the media using your needle, stabbing down the center of the media in the tube
* **significant results:**
15
New cards
IMViC tests
(**I**ndole, **M**ethyl Red, **V**oges-Proskauer, **C**itrate)
a battery of tests to help identify enterics and other GNRs
a battery of tests to help identify enterics and other GNRs
16
New cards
Negative staining
* Negative stains are when we use Nigrosin (an acidic stain → it has a neg. charge) to stain bacteria and view them in their original shape and size due to the lack of heat fixation. The negative charge of the stain repels the negative charge of the bacteria’s cell, making it appear clear.
* A negative stain is very good for observing cell morphology, arrangement, and size because the slide is NOT heat-fixed.
* A negative stain is very good for observing cell morphology, arrangement, and size because the slide is NOT heat-fixed.
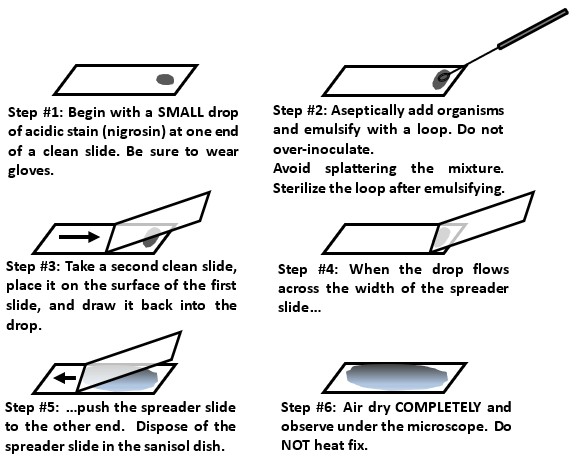
17
New cards
Simple staining
A simple stain is when we use a positively-charged dye to stain the negatively-charged bacteria and view them easily under the brightfield lens
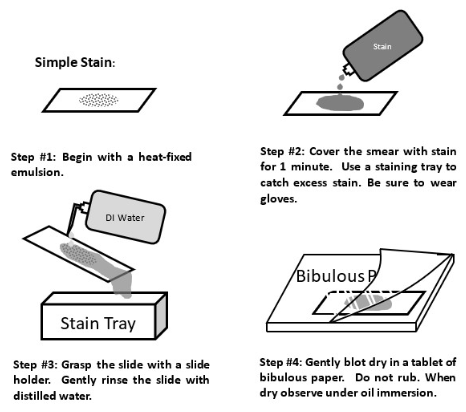
18
New cards
Gram staining
We might use a differential stain in a mixed sample of organisms to differentiate cell types. Bacterial cells with gram-positive cell walls are stained purple with the dye crystal violet, while bacterial cells with gram-negative cell walls are counterstained pink with safranin dye.
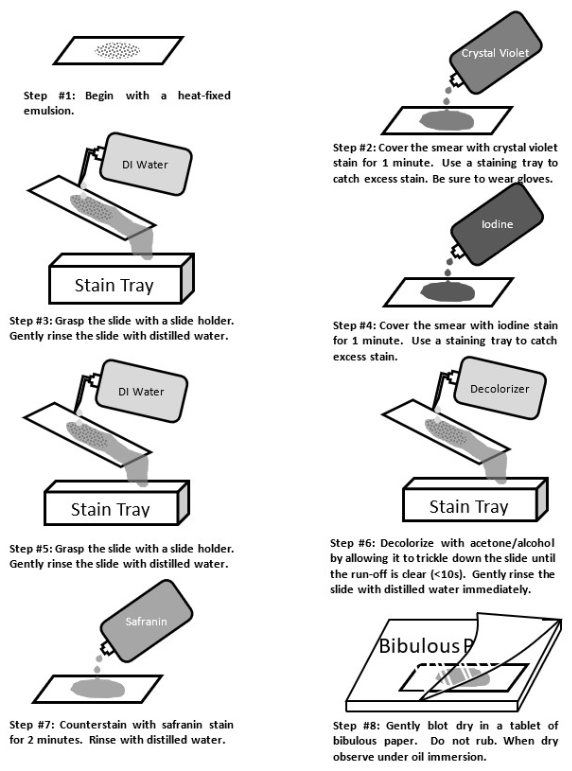
19
New cards
Endospore staining
* An endospore is a structure for the preservation of the DNA while conditions are poor
* The heating process makes the spore coat more permeable to the stain
* Endospores are formed when the bacterium is stressed.
* Endospores can be seen in simple and Gram stains, as a clear or empty space in a vegetative cell.
* The heating process makes the spore coat more permeable to the stain
* Endospores are formed when the bacterium is stressed.
* Endospores can be seen in simple and Gram stains, as a clear or empty space in a vegetative cell.
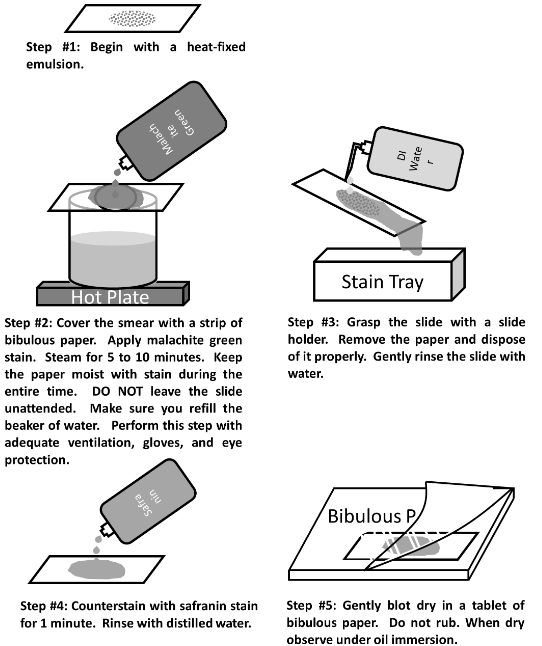
20
New cards
Acid Fast staining
* In the Acid-Fast stain, a very concentrated stain solution of kinyoun carbolfuchsin (hot pink) is used in order to penetrate and stain the cell walls
* This stain is a valuable diagnostic tool for finding tuberculosis and nocardial diseases in patient specimens
* Mycobacterium smegmatis is acid-fast positive
* This stain is a valuable diagnostic tool for finding tuberculosis and nocardial diseases in patient specimens
* Mycobacterium smegmatis is acid-fast positive
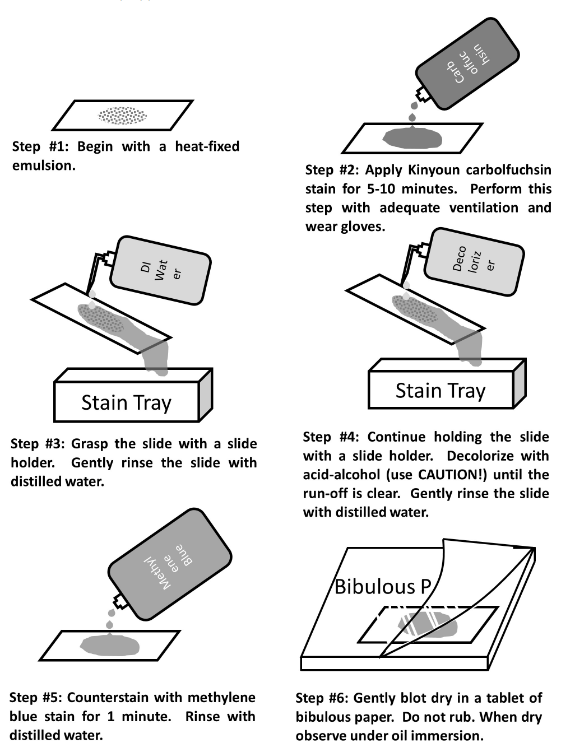
21
New cards
Smear prep from liquid or solid
(photo)
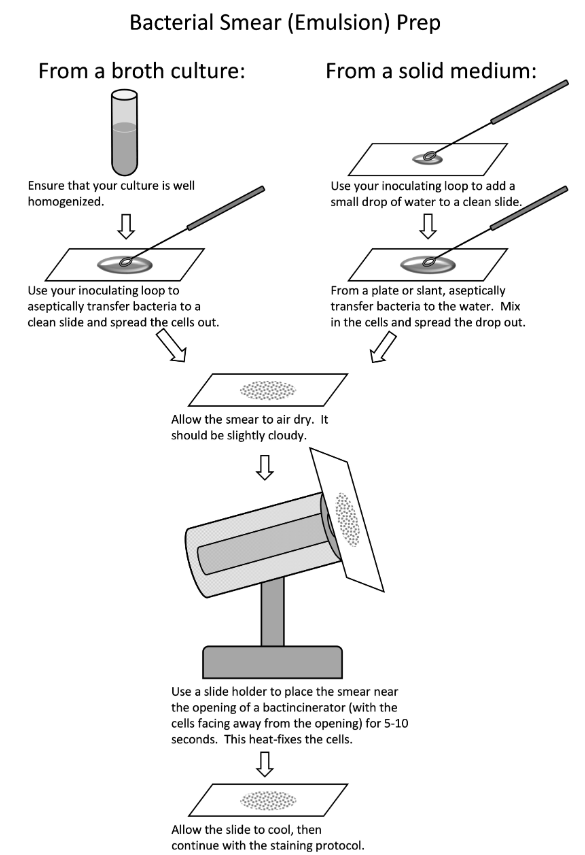
22
New cards
Good smear versus poor smear
A good smear is evenly distributed and not too thick or sparse.
If the smear is too thick the bacteria will not stain evenly. A thick/uneven smear cannot be properly decolorized and might wash off the slide despite fixation; we also won’t be able to see the bacteria clearly (morphology and arrangement) if they’re all piled on top of each other.
If the smear is too thick the bacteria will not stain evenly. A thick/uneven smear cannot be properly decolorized and might wash off the slide despite fixation; we also won’t be able to see the bacteria clearly (morphology and arrangement) if they’re all piled on top of each other.
23
New cards
Heat fixation
We use heat to fix the bacteria onto the slide so it doesn’t wash away with the staining agents and DI water.
24
New cards
Immersion oil
A drop of immersion oil is used ONLY on the 100x objective lens to view microorganisms more clearly since the oil increases the resolving power
Immersion oil has the same refractive index as glass
Immersion oil has the same refractive index as glass
25
New cards
How to observe differential stains
By always using brightfield on a 100X objective lens with oil immersion
26
New cards
What are the general characteristics of the protists?
eukaryotic and unicellular
27
New cards
Compare and contrast algae versus protozoa
* **Protozoa**: most are harmless free-living in a moist habitat but some are animal parasites; malaria and toxoplasmosis
* **Algae**: free-living; photosynthetic; causes Harmful Algal Bloom and Paralytic Shellfish Poisoning
* **Algae**: free-living; photosynthetic; causes Harmful Algal Bloom and Paralytic Shellfish Poisoning
28
New cards
What is the difference between a trophozoite and a cyst?
* **Cyst** = the dormant stage; very resistant structures that allow protozoa to survive in adverse environments
* **Trophozoite** = active feeding stage; absorbing nutrients from the host
* **Trophozoite** = active feeding stage; absorbing nutrients from the host
29
New cards
Why would we use a hanging drop slide?
preserves cell shape and arrangement
the Vaseline-sealed depression also slows down the drying-out process, so the organisms can be observed for longer periods.
the Vaseline-sealed depression also slows down the drying-out process, so the organisms can be observed for longer periods.
30
New cards
*Plasmodium vivax*
S.G. Chromalveolata, causes malaria, parasitic
31
New cards
*Giardia*
S.G. Excavata, flagella, diarrheal disease, parasitic
32
New cards
*Entamoeba histolytica*
S.G. Amoebozoa, pseudopods, diarrheal disease, parasitic
intestinal amebiasis, a **very common** protist infection worldwide, can survive hours in a pool and only needs 10 cysts to infect
intestinal amebiasis, a **very common** protist infection worldwide, can survive hours in a pool and only needs 10 cysts to infect
33
New cards
*Trichomonas vaginalis*
S.G. Excavata, flagella, STI, parasitic
**very common** STI; doesn’t form cysts so needs to go from “ideal” wet environment to the next via sex
**very common** STI; doesn’t form cysts so needs to go from “ideal” wet environment to the next via sex
34
New cards
*Cryptosporidium*
S.G. Chromalveolata, diarrheal disease, parasitic
can survive in a chlorinated pool
can survive in a chlorinated pool
35
New cards
What are the major characteristics of fungi?
chemoheterotrophic organisms that are usually nonmotile and grow by absorbing nutrients from their surroundings
36
New cards
Descriptions of molds versus yeasts
**Yeasts** are single-celled while **molds** are multi-celled (made up of hyphae)
37
New cards
Hyphae and mycelia
**hyphae** = long, threadlike filaments
**mycelia** = mass of hyphae
**mycelia** = mass of hyphae
38
New cards
Types of sexual spores - what are the differences?
Ascomycota → ascospores
Basidiomycota → basidiospores
Zygomycota → zygospores
Basidiomycota → basidiospores
Zygomycota → zygospores
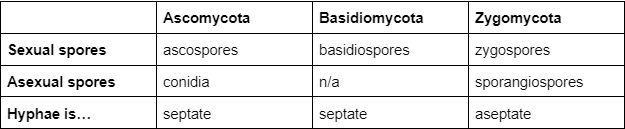
39
New cards
Types of asexual spores - what are the differences? (sporangiospores, conidia)
**conidia** = not in a sac, extend from the hyphae
**sporangiospores** = enclosed in a sac
**sporangiospores** = enclosed in a sac
40
New cards
*Candida albicans*
* Sexual: Ascomycota
* Asexual: budding?
* Lives on your body (skin, GI and vaginal tract, & oral cavity)
* dimorphic
* septate
* Candidal Intertrigo (superficial skin-fold infection) and *Candida* “Thrush” (overgrowth in the mouth and throat that appear as white, raised bumps)
* Asexual: budding?
* Lives on your body (skin, GI and vaginal tract, & oral cavity)
* dimorphic
* septate
* Candidal Intertrigo (superficial skin-fold infection) and *Candida* “Thrush” (overgrowth in the mouth and throat that appear as white, raised bumps)
41
New cards
*Coccidioides immitis*
* Sexual: Ascomycota
* Asexual: segmentation of hyphae produces arthrospores and can be inhaled?
* Windy conditions spread its fungal spores easily; septate
* dimorphic
* saprophytic
* septate
* Valley Fever
* Asexual: segmentation of hyphae produces arthrospores and can be inhaled?
* Windy conditions spread its fungal spores easily; septate
* dimorphic
* saprophytic
* septate
* Valley Fever
42
New cards
*Rhizopus*
* Sexual: Zygomycota
* Asexual: sporangiospores
* dimorphic
* saprophytic
* aseptate
* bread mold
* Asexual: sporangiospores
* dimorphic
* saprophytic
* aseptate
* bread mold
43
New cards
*Aspergillus*
* Sexual: Ascomycota
* Asexual: conidia
* Very common airborne soil fungus
* dimorphic
* septate
* “Farmer’s Lung”
* Asexual: conidia
* Very common airborne soil fungus
* dimorphic
* septate
* “Farmer’s Lung”
44
New cards
*Penicillium*
* Sexual: Ascomycota
* Asexual: conidia
* dimorphic
* septate
* Asexual: conidia
* dimorphic
* septate
45
New cards
General characteristics of the helminthes
They’re parasitic worms that are multicellular, eukaryotic, invertebrate animals, most are macroscopic but have microscopic life stages
* Phylum Platyhelmintes
* **Class Trematoda**
* flukes, leaf-shaped, intermediate hosts are mollusks, definitive hosts are vertebrae, digestive system
* **Class Cestoda**
* tapeworms, long/ribbon-like bodies, scolex for attachment, two or more hosts, no digestive system
* **Phylum Nematoda**
* roundworms, cylindrical, one host, complete digestive system, diverse and numerous
* Phylum Platyhelmintes
* **Class Trematoda**
* flukes, leaf-shaped, intermediate hosts are mollusks, definitive hosts are vertebrae, digestive system
* **Class Cestoda**
* tapeworms, long/ribbon-like bodies, scolex for attachment, two or more hosts, no digestive system
* **Phylum Nematoda**
* roundworms, cylindrical, one host, complete digestive system, diverse and numerous
46
New cards
What is a scolex and a proglottid?
The scolex is essentially the head, which contains organs which facilitate attachment to the host tissue
Proglottids are segments of the worm’s “neck” which contain eggs
Proglottids are segments of the worm’s “neck” which contain eggs
47
New cards
*Taenia solium*
Class Cestoda, proglottid, tapeworm
48
New cards
*Schistosoma*
Class Trematoda, blood fluke, can burrow into the skin, urine (and fecal) contamination, snails = intermediate host, not hermaphroditic, live in water
49
New cards
*Clonorchis sinensis*
Class Trematoda, oriental river fluke, parasitic, obtained by eating undercooked fish
50
New cards
*Enterobius vermicularis*
Nematoda, pin worm, **most common in U.S.**, mostly affects kids, very good at lasting in the environment; at night, the worm migrate to the anus and lay eggs
51
New cards
*Ascaris lumbricoides*
Nematoda, **most common in the world**, fecal/oral route, ingest egg → turns to larva → burrows out of intestine → pulmonary artery → lungs → clear throat → ingest again
52
New cards
*Necator americanus*
Nematoda, hookworm, live in humid/swampy areas, burrow into your skin (in between your toes)