chemistry - alcohols
1/52
Earn XP
Description and Tags
IGCSE Edexcel Alcohols (organic chemistry) flashcards
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
what is meant by functional group?
a group of atoms that determine the chemical reactions of a homologous series
what is meant by homologous series?
a series of compounds with the same functional group, general formula, similar chemical properties and trends in physical properties
what is the functional group of alcohols?
hydroxyl - all end in -ol
-OH
what is the general formula for alcohols?
CnH2n+1OH (separate to show fg), or CnH2n+2O
name the first four alcohols
methanol
ethanol
propan-1-ol
butan-1-ol
give the molecular formula of methanol
CH3OH (H4O)
give the molecular formula of ethanol
C2H5OH (H6O)
give the molecular formula of propan-1-ol
C3H7OH (H8O)
give the molecular formula of butan-1-ol
C4H9OH (H10O)
give the structural formula of methanol
CH3OH
give the structural formula of ethanol
CH3CH2OH
give the structural formula of propan-1-ol
CH3CH2CH2OH
give the structural formula of butan-1-ol
CH3CH2CH2CH2OH
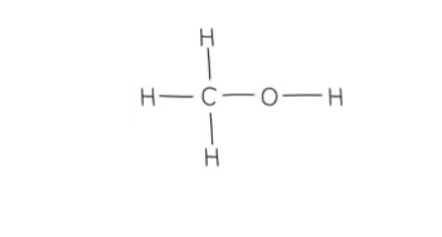
what is the displayed formula of methanol?
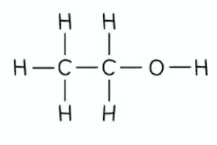
what is the displayed formula of ethanol?
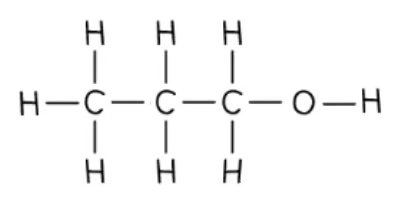
what is the displayed formula of propan-1-ol?
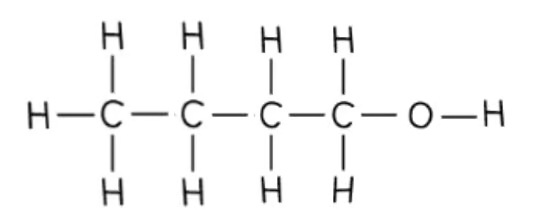
what is the displayed formula of butan-1-ol?
what is the word equation for the complete combustion of ethanol?
ethanol + oxygen → carbon dioxide + water
what is the chemical equation for the complete combustion of ethanol?
C2H5OH + 3O2 → 2CO2 + 3H2O
what are the two possible word equations for the incomplete combustion of ethanol?
ethanol + oxygen → carbon + water
ethanol + oxygen → carbon monoxide + water
what are the two possible chemical equations for the incomplete combustion of ethanol?
C2H5OH + O2 → 2C + 3H2O
C2H5OH + 2O2 → 2CO + 3H2O
what is produced when an alcohol is oxidised?
carboxylic acid + water
what are the two kinds of oxidation of ethanol?
microbial
chemical
what is the word equation for the microbial oxidation of ethanol?
ethanol + oxygen → ethanoic acid + water
what oxidising agent is required for microbial oxidation of ethanol?
bacteria (above arrow)
what is the chemical equation for the microbial oxidation of ethanol?
C2H5OH + 2[O] → C2H3OOH + H2O
what is the word equation for the chemical oxidation of ethanol?
ethanol + oxygen → ethanoic acid + water
what conditions are needed for the chemical oxidation of ethanol?
presence of potassium dichromate (IV) (K2Cr2O7)
presence of dilute sulfuric acid
heat
what colour change is seen when ethanol is oxidised during chemical oxidation?
orange → green
what is the chemical equation for the chemical oxidation of ethanol?
C2H5OH + 2[O] → C2H3OOH + H2O
what two processes are there for the production of ethanol?
hydration
fermentation
what is the word equation for fermentation?
glucose (aq) → ethanol + carbon dioxide
what is the chemical equation for fermentation?
C6H12O6 → 2C2H5OH + 2CO2
what conditions are required for fermentation?
anaerobic
yeast - catalyst
30OC
why are anaerobic conditions required for fermentation?
so that the yeast respires anaerobically to produce ethanol otherwise ethanol would not be produced if oxygen was present
why is a temperature of 30C required for fermentation?
not too high so enzymes don’t denature and the reaction stops happening (slows)
not too low so the rate of reaction is high enough
what can be used as a source of glucose?
sugar cane
what is the word equation for hydration?
ethene (g) + water (g) → ethanol (g)
what is the chemical equation for hydration?
C2H4 + H2O → C2H5OH
what conditions are required for hydration?
300C
65 atm (pressure)
phosphoric acid catalyst (H3PO4)
is hydration a reversible reaction?
yes
what type of resources does fermentation use?
renewable, cheap resources
what type of resources does hydration use?
finite, non-renewable resources
what type of process is fermentation?
batch process - inefficient - takes a long time
what type of process is hydration?
continuous process - more efficient
what is the quality of product like for fermentation?
impure - contains CO2 - needs distillation as it is unconcentrated and purification
what is the quality of product like for hydration?
pure
what is the rate of reaction for fermentation?
slow - days
what is the rate of reaction for hydration?
fast
what is the energy usage like for fermentation?
low - needs low temperature and low pressurer
what is the energy usage like for hydration?
high and thus also expensive - needs extremely high temp and pressure
what is the atom economy for fermentation?
makes by product
what is the atom economy for hydration?
makes only desired product