3: Protein function
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
How many peptides make up hemoglobin?
4 - tetramer
How many peptides make up myoglobin?
1 - monomer
What does myoglobin do?
store oxygen in muscles
release oxygen when rapidly contracting muscle need energy (ie. O2 levels drop)
What does haemoglobin do?
transport oxygen
responds to O2 concentration, blood pH, regulators
take back CO2
What are myoglobin and hemoglobin called?
homologues - more specifically paralogues
What are paralogues?
closely related
same folds
similar sequence identity
Describe the structure of myoglobin and haemoglobin
each subunit of haemoglobin is similar to myoglobin
myoglobin is a single chain that folds into a globin fold, haemoglobin has four globin folds
heme group - contains an iron II center (allowing oxygen to bind)
Describe the structure of haemoglobin
tetramer (four parts)
dimer of two alpha-beta ‘protomers’
What is a protomer?
structural unit of a protein with a quaternary structure ie. aplha/beta pair in haemoglobin
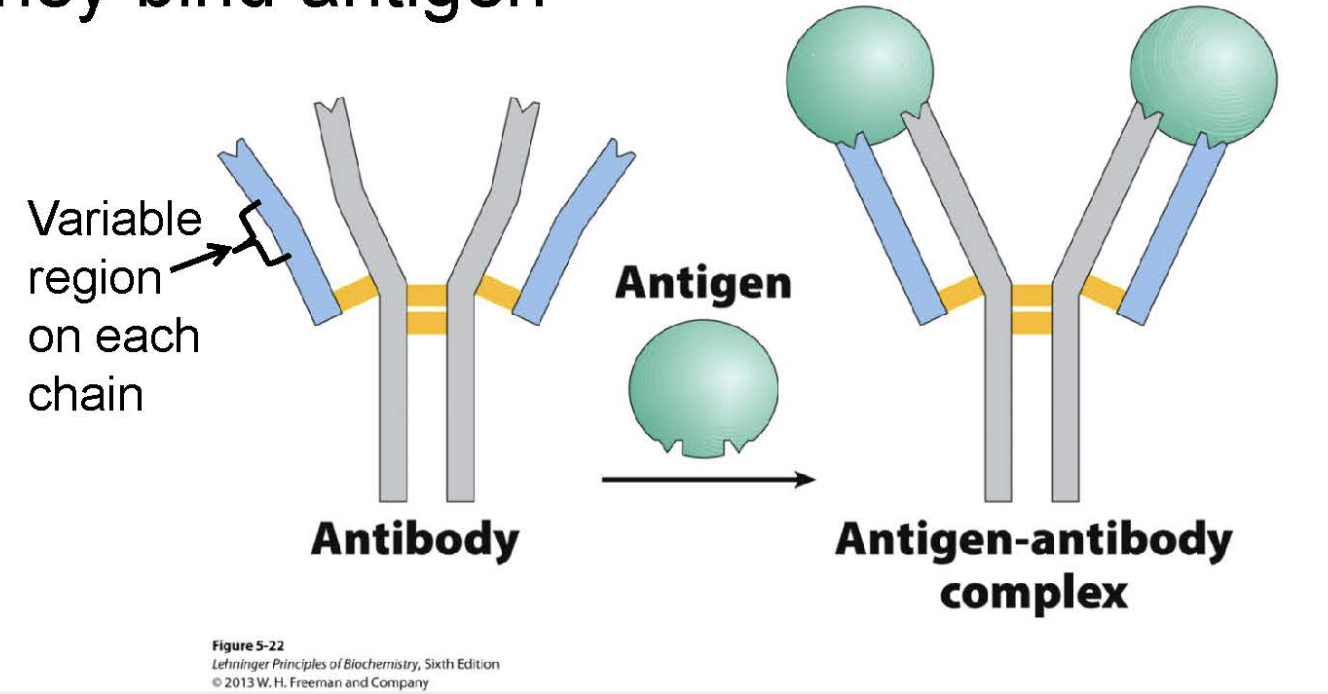
What is an example of quaternary structure and describe the structure and function
immunoglobulin Gs (IgGs)
four subunits: 2 heavy 2 light chains
involved in binding to antigen ligand
variable region on each chain undergoes conformational change when bound
2 antigens can bind
immunoglobulin fold
Why do antibodies have quaternary structure/multivalent?
more efficient binding
if one fails, still have backup
What is multivalency?
ability to bind to multiple things
Why does haemoglobin have four binding sites/quaternary structure?
allows for more oxygen to bind
allosteric cooperative binding
Is myoglobin more abundant in land or sea animals?
animals which dive
What colour does myoglobin give to muscles?
increased myoglobin = increased dark red muscle
ligand vs binding site
oxygen = ligand
myoglobin/protein = binding site
What is the prosthetic group?
non-protein that is bound separately to the protein ie. heme group in myoglobin
Eqm association vs. eqm dissociation constant
Ka vs Kd
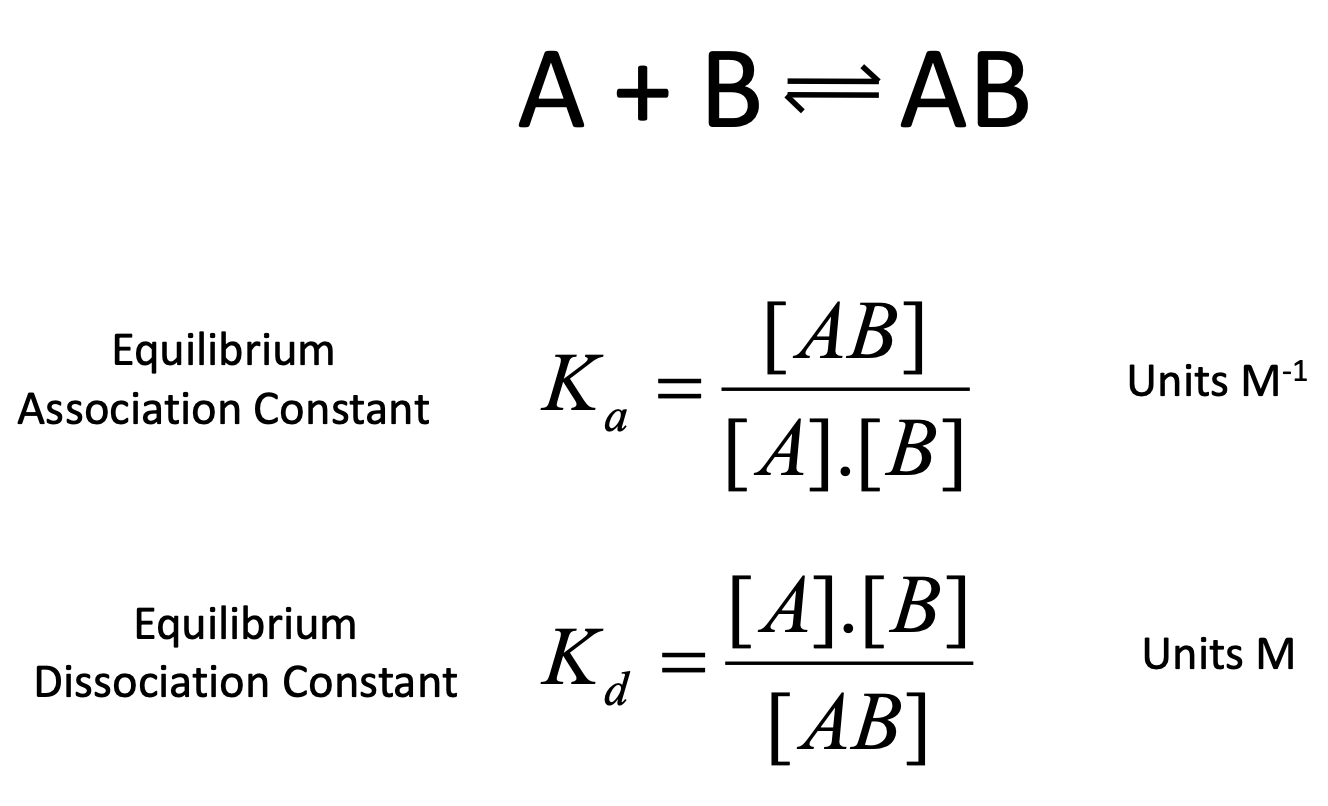
is Kd or Ka preferred in biosciences?
Kd as it is useful in expressing ligand binding as it represents the conc. of free ligand at which protein is 50% saturated
high affinity vs low affinity of Kd values
high = 1 × 10-15 M
low = 1 × 10-2 M
What is the highest affinity? Why?
biotin
Can myoglobin transport O2?
binds oxygen very tightly (high pressure in lungs)
will not release it (low pressure in tissues)
Why is haemoglobin so good at what is does?
conformational state changes (tight to retain O2, loose to release)
for effective transport, affinity must vary with pO2
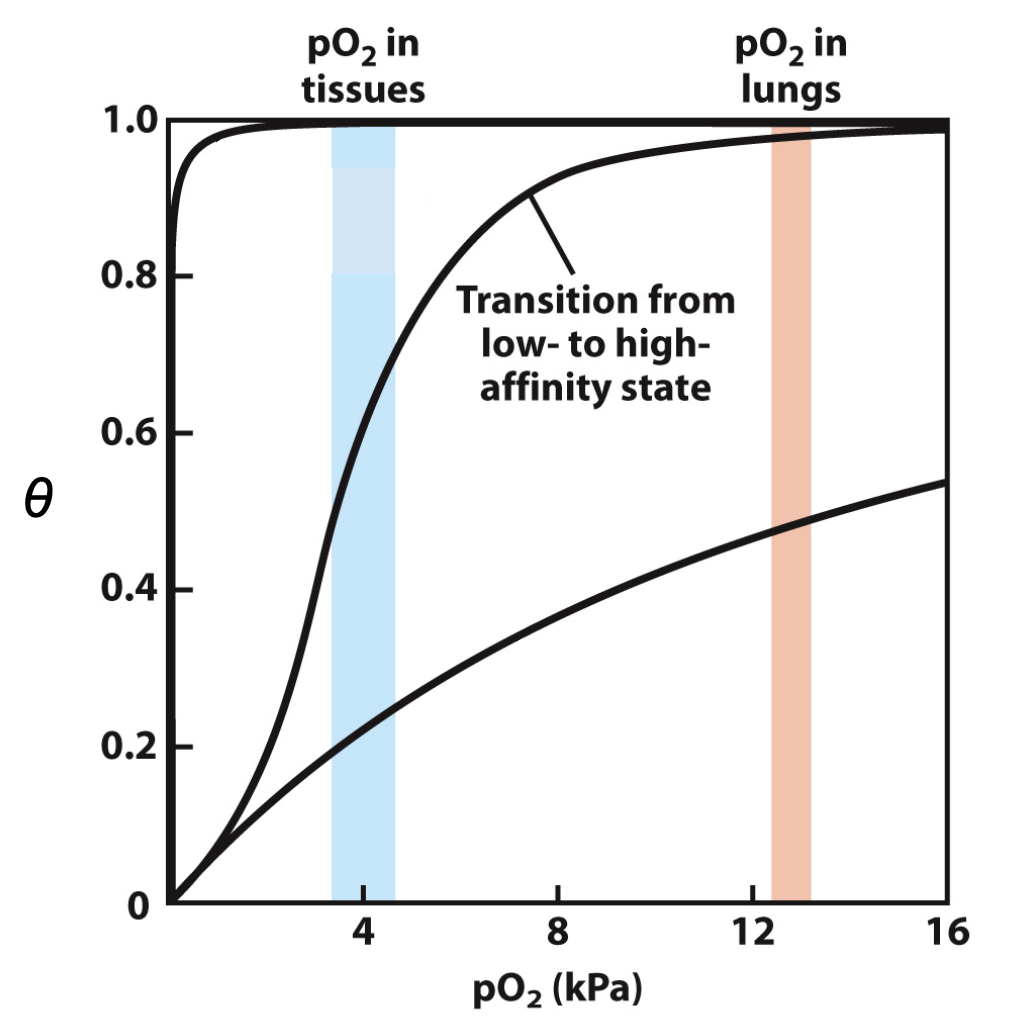
R and T states
T = tense state
more interactions, more stable
lower affinity for O2
stabilised by variety of salt bridge interactions
R = relaxed state
fewer interactions, more flexible
higher affinity for O2
How does O2 binding affect haemoglobin?
O2 binding triggers T→R conformational change
breaking salt bridges b/w residues at alpha1-beta1 interface
T = His outside
R = His inside
What stabilises T state of haemoglobin? What destabalises it?
salt bridge between His HC3 (last residue of beta subunit) and Asp FG1
also between Arg and Asp in alpha subunits
and C terminus of alpha 1 and N terminus in alpha 2
destabilised by O2 binding - via flattening of heme group and breaking of salt bridges
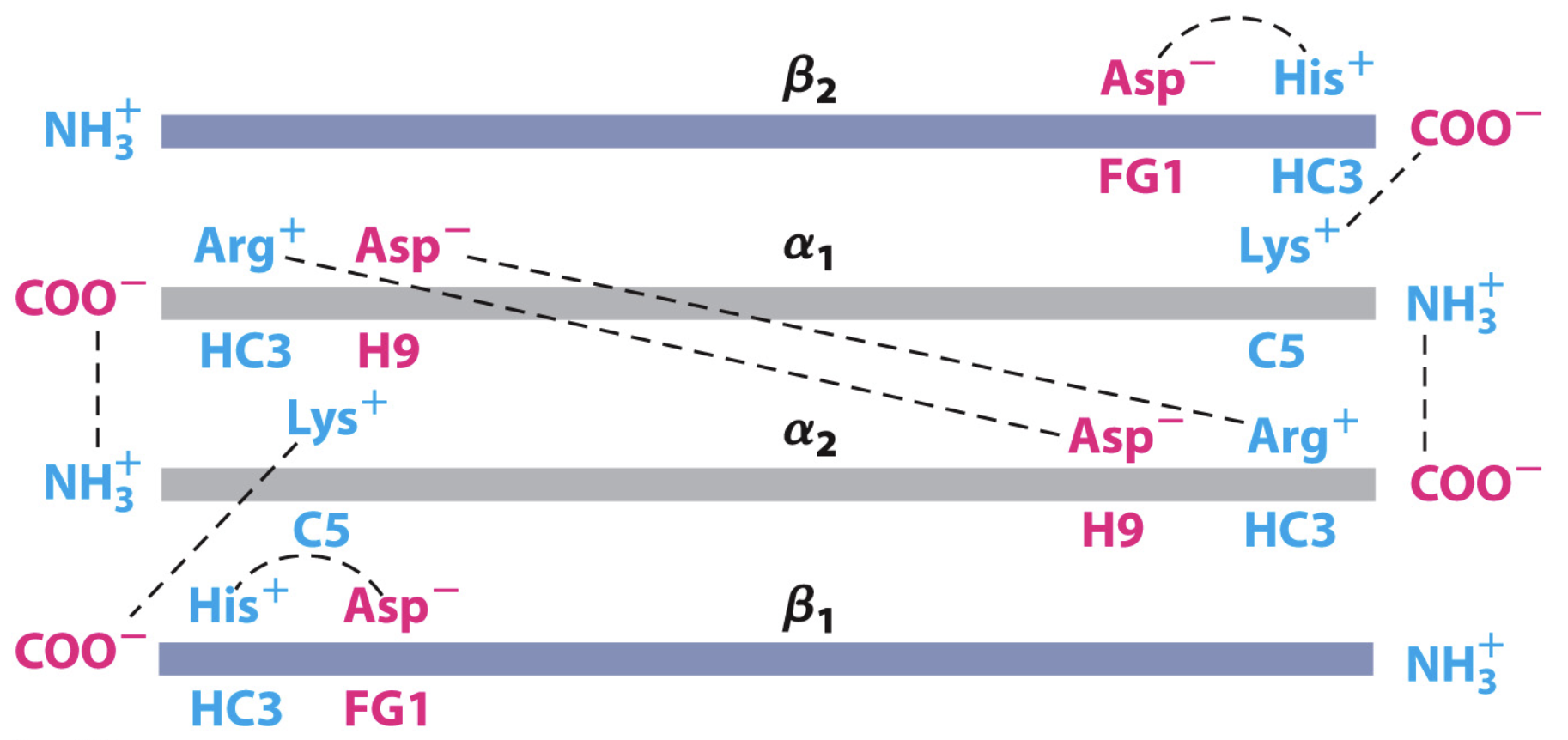
What does HC3 and FG1 mean?
third residue, C-terminal to helix H
first residue between loop F and G helices
What is the Hill equation?
theta = fraction of binding sites occupied
L = free ligand
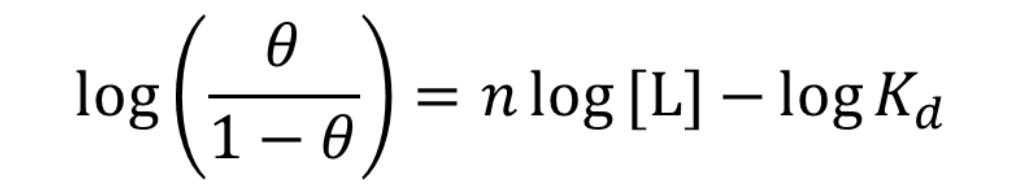
What is a Hill plot?
y vs. x
What does the slope of a hill plot show?
measure of the degree of interaction (ie, the degree of 'cooperativity') between binding sites
indicates the interaction between binding sites rather than the actual number of binding sites
What is the Hill coefficient?
the slope of the hill plot nH
What does nH > 1mean?
positive cooperativity
binding at one site increases binding at other sites
eg. Hb and O2
What does nH = 1mean?
binding is not cooperative
sites are independent
What does nH < 1mean?
RARE
negative cooperativity
binding at one site decreases binding at other sites
What is the theoretical upper limit of nH?
n, experimentally however it is almost always lower ie. < n
What is nH related to?
average occupancy of the binding sites not total
What are the models of cooperativity?
concerted and sequential
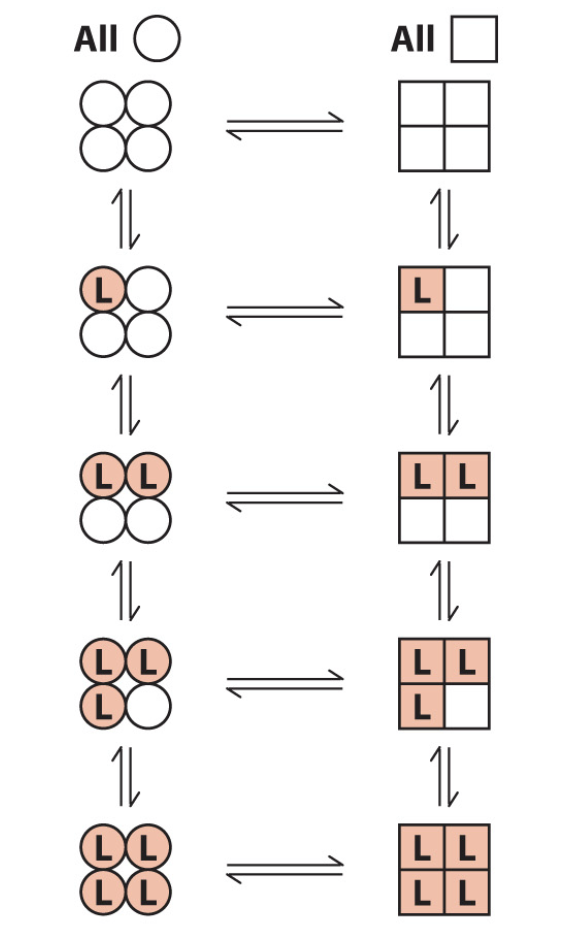
What is the concerted model of cooperativity?
all or nothing
in the absence of L, all subunits of a multimer are thought to be in the inactive T (more stable) or the active R form
circles = T, squares = R, shading and L = ligand binding
inactive state destabalised by L binding
all subunits transition from T to R simultaneously
What is the sequential model of cooperativity?
each subunit of the multimer can be in either the T or R form
L binding procedures a change in conformation of the subunit
a change in confirmation in one subunit induces a similar change in an adjacent subunit
therefore binding of a second L is more likely
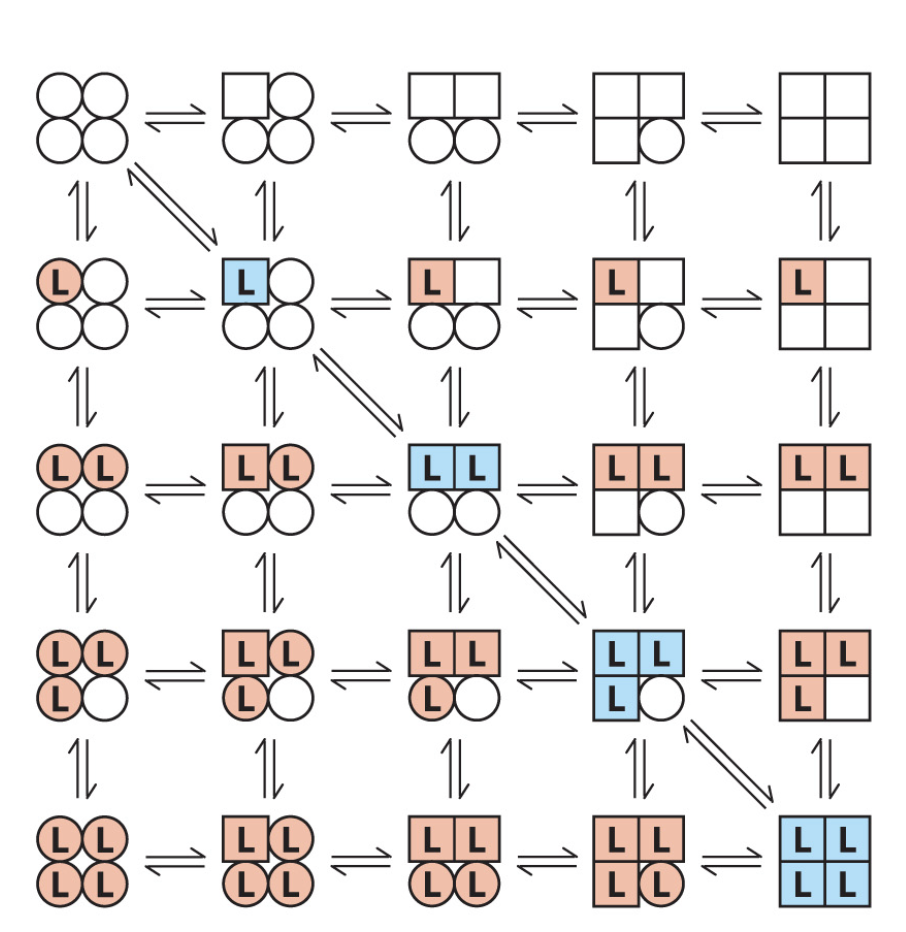
Are the two models of cooperativity mutually exclusive?
NO! the sequential one has concerted in it.
What are the end products of metabolism in tissues?
H+ and CO2
how much of H+ and CO2 does Hb transport?
40% of tissue H+ and up to 20% of CO2 to lungs and kidneys
True or False: H+, CO2 and O2 are all transported in the same way?
False! O2 is not transported the same way as H+ and CO2
True or False: H+, CO2 and O2 are all competing to bind to the heme group??
False!
How is H+ produced?
metabolism directly
when CO2 reacts with H2O to form HCO3-
How does pH affect O2 binding to Hb?
lower pH (higher [H+]) = lower affinity = help offload O2
higher pH = higher affinity = retention of O2
What are actively metabolising tissues generates?
H+ - lowering pH of blood
What happens when H+ binds to Hb?
stabalises T state
protonates His HC3 → forming a salt bridge with ASP FG1
release of O2 into the tissuesf
What is the Bohr effect?
the pH difference between lungs and metabolic tissues increases efficiency of the O2 transport
How are protons (H+) transported?
N-terminal of alpha-subunits
His146 (His HC3) of the beta subunit
other amino acid residues
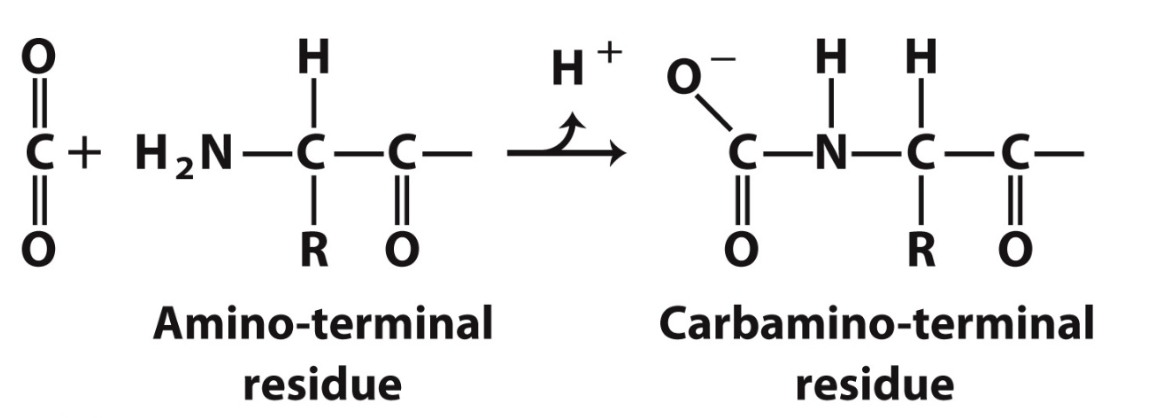
How is CO2 transported?
15-20% as carbamate on amino terminal residues
yields a proton → Bohr affect
forms additional salt bridges, stabilising T state
CO2 release in lungs favours the R state
What is BPG?
2,3-bisphosphoglycerate
derived from intermediate in glucose metabolism
highly negative
stabilises T state
Where does BPG bind?
on Hb at a seperate binding site to O2 but still affects O2 binding
allosteric
at the cavity in the middle of T state
Where is BPG found?
in RBCs
True or False: BPG is an allosteric regulator of Hb?
True! affects binding of O2 without binding to O2 site
What is the best stabiliser for T state?
BPG
What does BPG allow for?
O2 release in the tissues
adaptation to changes in altitude
Where is increased BPG => Hb bind O2 more weakly advantageous?
high altitude
At sea level, about 38% of O2 in saturated Hb (in the lungs) is delivered to the tissues
At high altitude, pO2, in lungs decreases so Hb is less saturated and O2 delivery decreases to 30%
What binds to Hb and where?
H+, BPG and CO2
all at different ALLOSTERIC sites
What do H+, BPG and CO2 do to Hb?
stabilise T state (by different mechanisms to lower affinity of Hb for O2
What do allosteric affects of H+, BPG and CO2 give rise to?
cooperativity of the binding due to changes in the conformation that are transmitted through the subunits of Hb
What can you call H+, BPG and CO2?
ligands
Is CO or CO2 more toxic? Why?
CO
CO2 is transported in a different way than O2 (as carbonate ion)
CO can take up O2 binding sites - heme group → and much more tightly → competitive inhibitor
Does CO stabilise Hb like O2?
Yes! stabilises R state just like O2 does