electrons in atoms unit 3
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
describe light
light is an electromagnetic wave which carries energy. different colors of light correspond to different wavelengths and frequences
describe the order for wavelength
decreasing to increasing
gamma rays
x-rays
rainbow (violet to red)
infrared
microwave
radio
describe the order for energy
decreasing to increasing
radio
microwave
infrared
rainbow (red to violet)
x-rays
gamma rays
describe the order for frequency
decreasing to increasing
radio
microwave
infrared
rainbow (red to violet)
x-rays
gamma rays
describe frequency
Frequency, ν, is the number of peaks passing the observation point per unit time. The SI unit of frequency is Hz, (1 Hz = 1 s‒1 ).
describe wavelength
Wavelength, λ, is the distance between peaks measured in m. Nanometers (1 nm = 10‒9 m) are often used in the case of visible light.
whats the equation for speed of waves
c (speed of light)= wavelenght * frequency
describe the connection between frquency wavelgnth and energy of light
Frequency and wavelength of light are inversely proportional
The energy of light is proportional to frequency. 𝐸 = ℎv
describe diffraction
the bending of waves around corners. Constructive and destructive interference patterns.
describe refraction
the bending of light as its speed changes in passing from one medium to another
what is blackbody radiation
all objects emit electromagnetic radiation due to thermal energy (except at 0 K)
what is the ultraviolet catastrohpe
Classical wave theory could not predict observed spectra
describe what max planck did
Planck explained the observed spectrum – its exact shape – by postulating that light energy is quantized.
The smallest increment of light energy is the energy of a single particle of light, a photon.
The energy of one photon of light with frequency, ν, is given by 𝐸𝑝ℎ𝑜𝑡𝑜𝑛 = ℎ𝜈 where h = 6.62606957×10‒34 Js is called Planck’s constant
what does the voltmeter do
Voltmeter → measures how fast the e- are going (Kinetic Energy)
what does the ammeter
how many e- are ejected and travelling (Current)
describe the photoelectric efffect
Sufficiently high frequency/energetic photons eject electrons from the surface of a metal.
Ephoton > Ethreshold
Ethreshold = work function of the metal, symbol Φ
Φ changes with different metals
what section has the smallest work function
Alkali metals have the smallest work functions - it takes less energy to eject an electron from an alkali metal.
According to decreasing work function, lithium > sodium > potassium > rubidium > cesium.
describe the work function
If Ephoton > Φ, then the excess energy goes towards the kinetic energy of the ejected electron
Exactly one electron is ejected from the metal surface for every one photon of light (provided it is energetic enough).
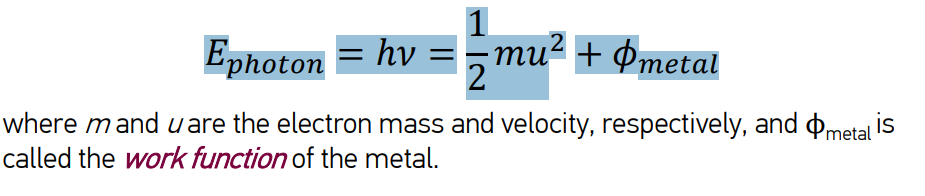
describe daltons atom
single, indivisible entity unique for each element, cannot be altered or destroyed and combines to more complex compounds
describe plum pudding model
negative charged “corpuscles” (electrons) embedded in a sea of positive charge
describe nucluer model
highly concentrated positive charge in a very small volume which contained the bulk of the atom’s mass surrounded by orbiting electrons
describe energy of electron transition
The difference between two of these energies determines a transition energy equal to the energy of the photon emitted or absorbed.
E > 0: absorption of a photon
E < 0: emission of a photon
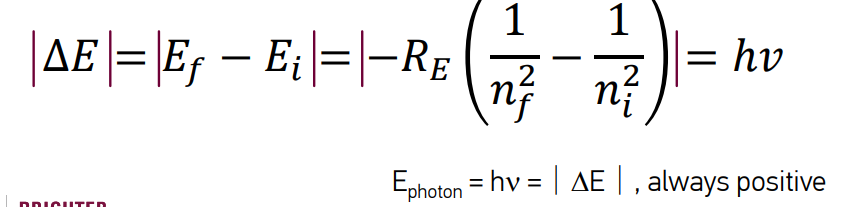
describe what de broglie did
Electrons & nuclei, & light are all treated as wave-like & particle-like
de Broglie first suggested that particles, such as electrons, are wavelike, characterized by a wavelength
The wavelength of matter (in meters) is equal to the ratio of Planck’s constant over the product of the mass (in kg) times the velocity (in m/s).
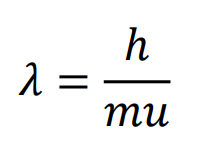
what is the Heisenberg Uncertainty Principle
There is uncertainty in the position of an electron in an atom.
One can only speak of the probability of finding the electron in a volume within the atom.
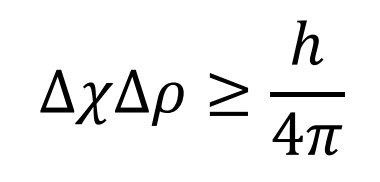
describe the wave function
The solutions to Schrodinger’s Equation correspond to a wave function, Ψ, which (together with the spin) describes the state of the electron in the atom as a wave
The square of the wave function, Ψ2 , at each position gives the likelihood of finding the electron in that location.
The region of space where we find an electron 90% of the time defines the boundaries of the orbital
describe the pinicpal qunatum number (n)
The Principal Quantum Number describes the energy and distance of the electron from the nucleus
It is analogous to the energy levels in the Bohr Model of the Atom
The greater the value of n, the further the electron is from the nucleus and the more energy it has
n=1,2,3,…
describe angular momentum number (ℓ)
Because of the spherical symmetry of the hydrogen atom (and electron spin), there is more than one state for each energy level.
The electron in hydrogen has an orbital angular momentum associated as it orbits about the proton nucleus.
Angular momentum (azimuthal) has a magnitude, with associated quantum number - angular momentum quantum number
ℓ = 0, 1, 2, …, n ‒1
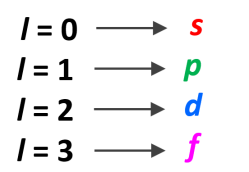
describe magnetic quantum number (mℓ)
Angular momentum has an orientation-associated quantum number - magnetic quantum number
Orbitals with the same angular momentum number are degenerate in energy
mℓ = ‒ℓ, ‒ℓ + 1, …, ℓ ‒1, ℓ
describe s orbitals
The ℓ = 0 orbitals have spherical shape. m ℓ = 0
Between nodes is a radial node
The ns orbital has n – 1 radial nodes.
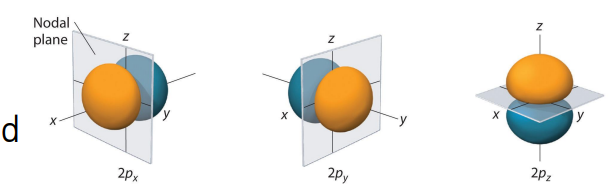
describe p orbitals
The ℓ = 1 orbitals have dumbell shape.
Three degenerate np orbitals mℓ = -1, 0, +1
Angular nodes between positive and negative lobes
The np orbital has n – 2 angular nodes.
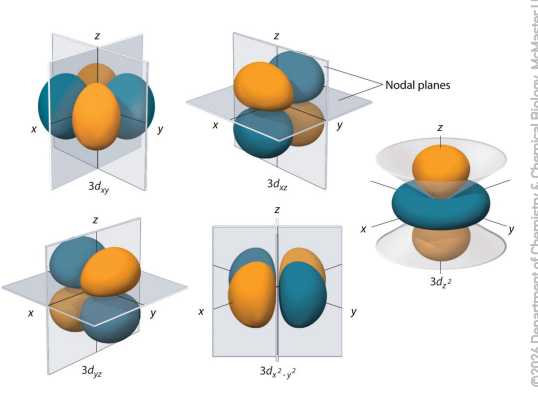
describe d orbitals
The ℓ = 2 orbitals have clover shape.
Five degenerate np orbitals mℓ = -2, -1, 0, +1, +2
Angular nodes between positive and negative lobes
describe the spin quantum number
In addition to the orbital quantum numbers already introduced, the electron has a spin quantum number,
ms = ½ or ‒½, spin angular momentum pointing up or down, respectively.
There are two states associated with the lowest energy level: (n, ℓ, mℓ , ms ) = (1, 0, 0, ½) and (1, 0, 0, ‒½)
describe one electron systems
Ions with only one electron – i.e., He+ , Li2+,…, have the same orbitals as hydrogen, except that they held more closely to the nucleus by the larger nuclear charge.
ex. The energy levels of He+ are four times deeper than those of H because the nuclear charge is doubled.
describe multielectron systems
Electron repulsion makes calculating the states and energy levels difficult – computers approximate this
Each electron is assigned to an orbital
describe Pauli Exclusion Principle
two electrons with the same spin cannot occupy the same orbital.
describe Ground State Electron Configuration
set of occupied orbitals filled in order of increasing energy
Aufbau’s Principle – the occupation of electrons in order of increasing orbital energy
descirbe nucleur shielding
For one electron species, the greater the nuclear charge (Z), the lower the energy of the electron.
For multielectron species, electrons repulsions destabilize the electrons (shielding – S) and increase their potential energy
describe the effective numcleur charge equation
Inner electrons shield outer electrons from the full attractive charge from the nucleus (effective nuclear charge) Zeff = Z – S
Orbitals that penetrate closer to the nucleus experience a higher effective nuclear charge and reduced shielding (s > p > d > f)
what is hunds rule
electrons occupy degenerate orbitals singly first to avoid repulsions (pairing energy)
describe the Electron Configuration Exceptions

describe paramagnetic
atoms with unpaired electrons that are attracted to magnetic fields
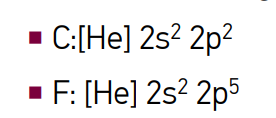
describe diamagnetic
atoms with entirely paired electrons that are weakly repelled by magnetic fields
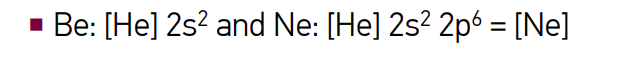
describe the devlopement of the periodic table
Dmitri Mendeleev (1834 – 1907 CE) formulated the Periodic Law and the Periodic Table of Elements which predicted elements yet discovered
Mendeleev organized the known elements into eight groups, in order of increasing mass. He left gaps for undiscovered elements and predicted their properties