Chapter 15: Signaling and signal transduction
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
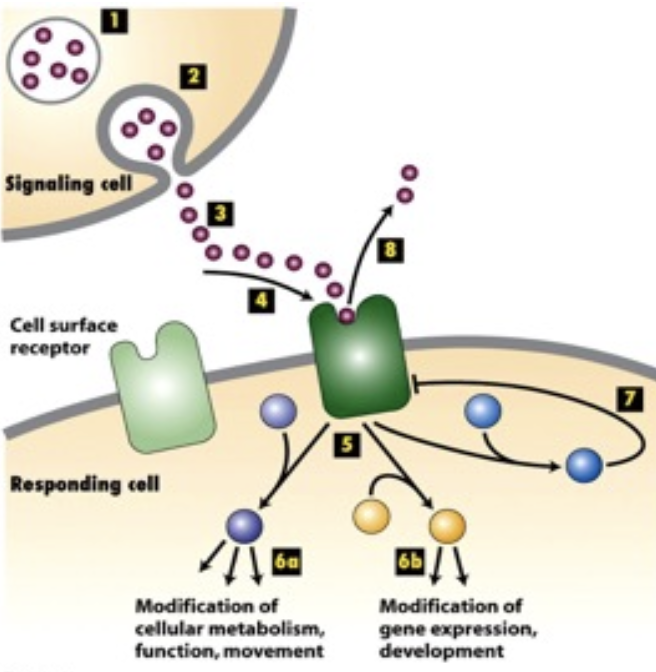
Whats the 8 basic elements/steps of cell signaling (Extracellular signal to cellular response)
These components work together to relay signals from the outside to inside a cell, facilitating communication and response:
synthesis of signaling molecule
release of signaling molecule via exocytosis
transit of signaling molecules to the target cell
binding of signaling molecule (ligand) to a protein receptor on the target cell
binding of ligand to receptor results in a conformational change of the receptor
receptor initiates one ore more intracellular pathways that results in changes in:
cellular function
metabolism
gene expression
shape
movement
deactivation of the receptor
removal of ligand to terminate the signal.
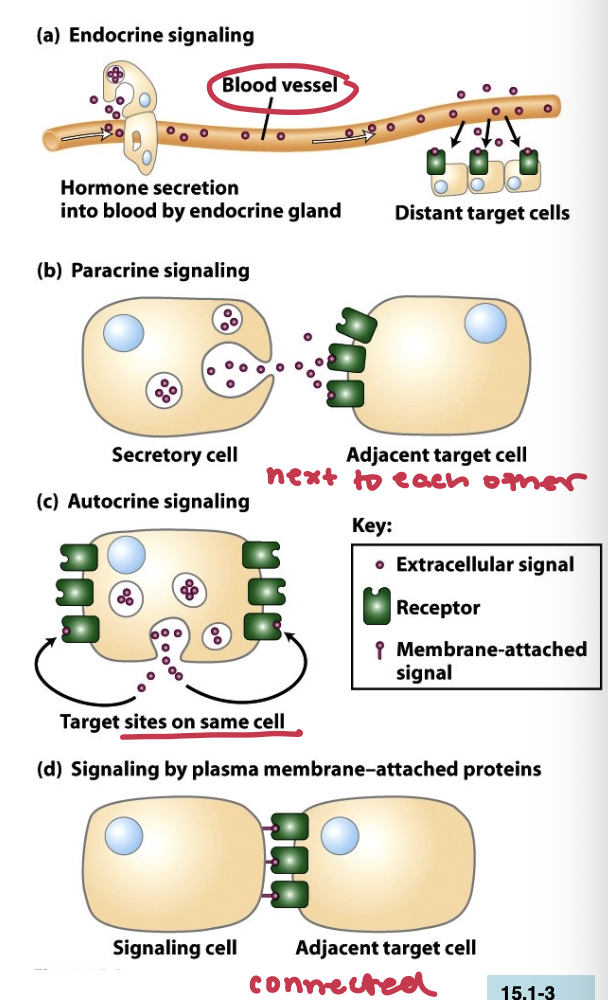
What’s the 4 categories of intracellular signaling signals
Each type refers to the way signals are transmitted and received within or between cells:
Endocrine signaling, messanger molecules reach their target cells through the bloodstream
insulin
Paracrine signaling, messenger molecules travel short distances through extracellular space
neurotransmitters
Autocrine signaling, the. cell has receptors on its surface that respond to the messenger 9
T-cells during an immune repsonse
Juxtacrine singaling (or contact dependent signaling), short range but requires physical contact between sending and receiving cells
antigen presentation
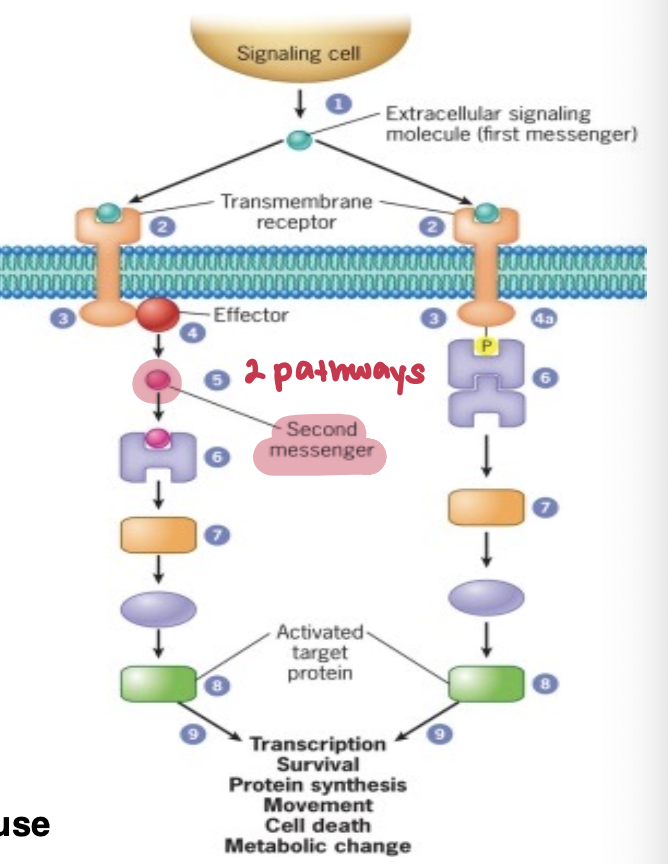
What’s the difference between first messenger and second messenger
First Messenger = ligand, are the extracellular signaling molecules that bind to receptors on the target cell.
Second Messenger = small molecules that increase or decrease in concentration in response to first messenger (can be anywhere along the cascade)
They bind to other proteins to modify their activity
Never second in a pathway but is called that b/c they were found after the ligand
Example for this course would be 3’,5’-cyclic AMP (cAMP) — activates protein kinase A (PKA)
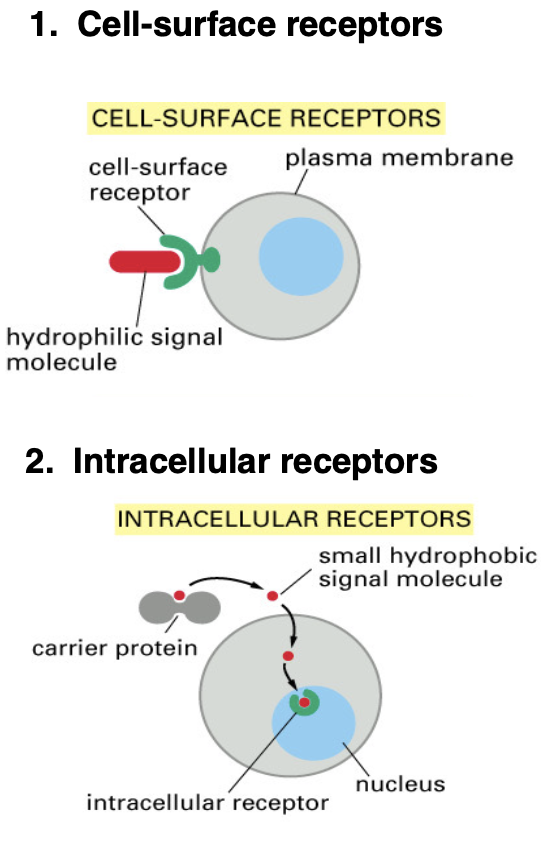
What are the 2 receptor locations for signaling
Cell-surface receptors = located on the cell membrane, bind to hydrophilic signaling molecules (hydrophilic so it can’t cross the plasma membrane)
Three main classes of these receptors: G-protein coupled (GPCR), Enzyme-linked, and Ion Channel-linked
Intracellular receptors = located inside the cell, bind to hydrophobic or small signaling molecules that can diffuse through the cell membrane and bind to receptor proteins either in the cytoplasm or nucleus
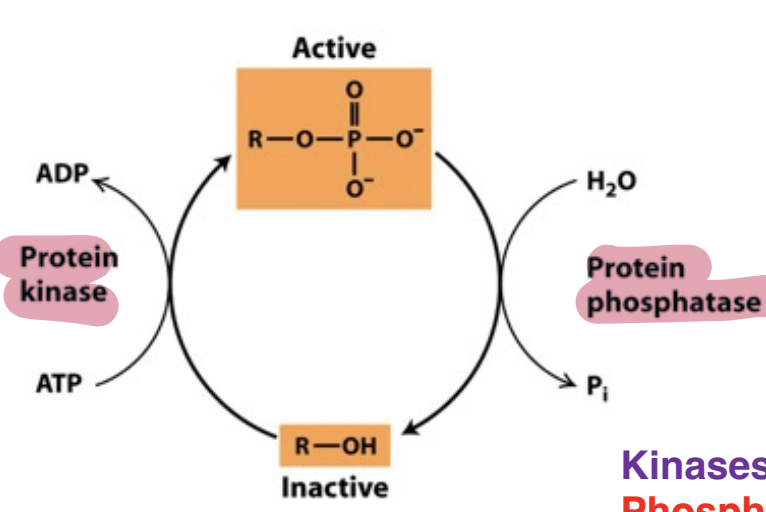
What’s the principles of regulation by phosphorylation and dephosphorylation
These dynamic processes plays a critical role in cellular signaling and regulation:
Phosphorylation = changes a proteins charge and generally leads to a conformation change which can alter ligand binding or other features of the protein resulting in an increase OR decrease of it’s activity (activate or deactivate functions)
I part of almost all signaling pathways
kinases phosporylate
Dephosporylation removes phosphate groups from proteins, reversing phosphorylation effects and thus modulating activity.
phosphatases dephosphorylate
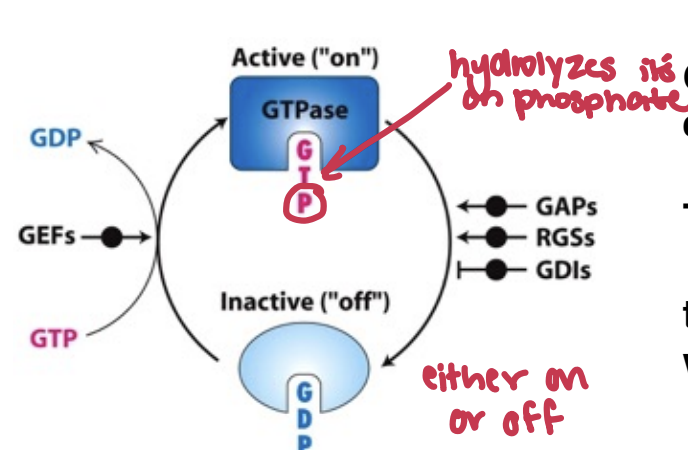
What’s the principles of GTPases and G proteins
GTPases (Small) are enzymes that hydrolyze GTP to GDP, functioning as molecular switches in signaling pathways. They toggle between active and inactive states to regulate various cellular processes, including signal transduction and cell growth.
enzyme on itself — happens to turn it off and is a instruction from something
all have very important functions
G proteins (Large) are guanine nucleotide-binding proteins that act as molecular switches in signaling pathways, mediating the transmission of signals from receptors to target effectors by cycling between active (GTP-bound) and inactive (GDP-bound) states.
heterotrimeric g-proteins the alpha subunit is identical to gtpase in structure and function
What are the roles for the components of Molecular Switches (GTPase, GAPS, RGSS, GDI, GEFS)
Molecular switches, such as kinases and phosphatases, regulate signaling pathways by adding or removing phosphate groups, influencing protein activity.
GTPase: enzymes that hydrolyze GTP to GDP
on = bound GTP
off = bound GDP
GAPs: GTPase-activating proteins
RGSs: regulators of G protein signaling,
GDI: guanine nucleotide dissoication inhibitors
GEFs: guanine nucleotide exchange factors
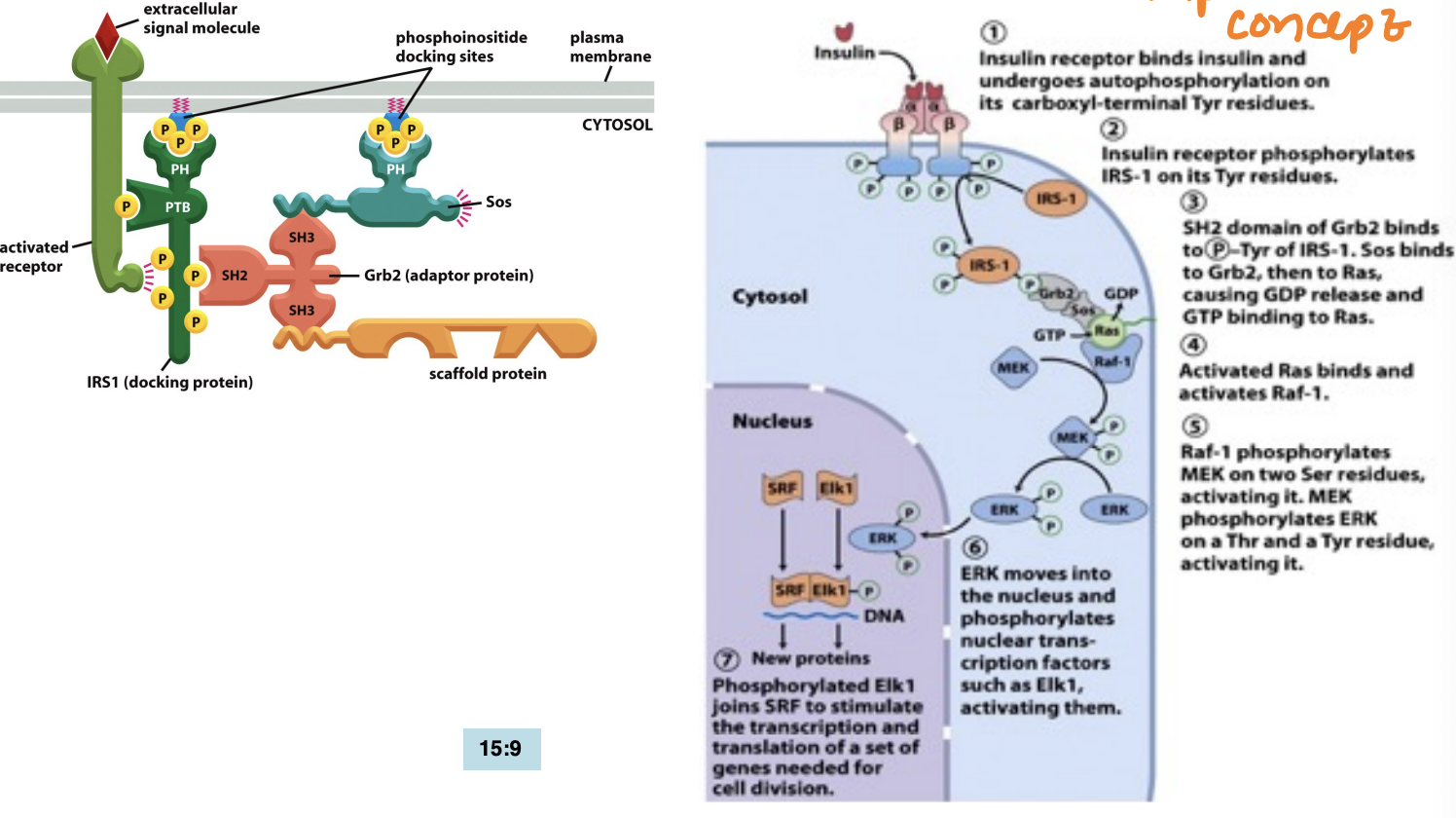
What’s the principle of protein domains in docking (PTB, SH2, SH3)
Protein domains like PTB, SH2, and SH3 facilitate the specific interactions between signaling proteins by recognizing and binding to phosphorylated tyrosines or proline-rich motifs, playing crucial roles in signal transduction.
similar structural domains and allow for recruitment of signaling complexes to activate downstream pathways.
They bind to things (like phosphorylated tyrosine, yellow dots and amino acid sequences)
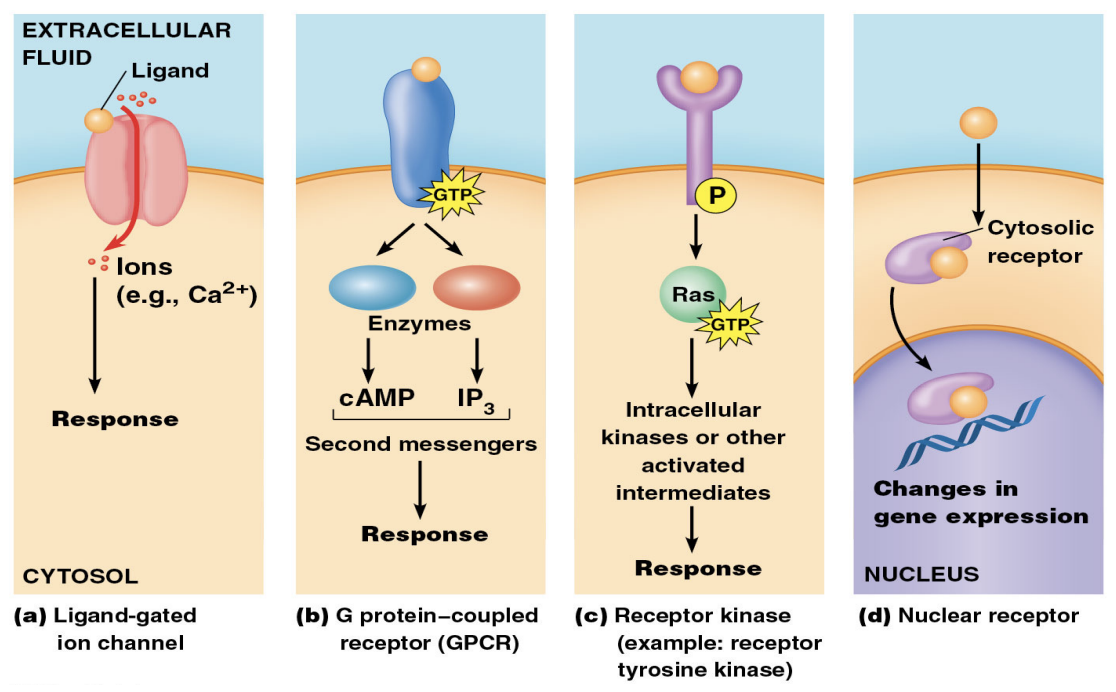
4 types of extracellular messengers and their receptors
Ligand-gated ion channel: conduct a flow of ions across the plasma membrane to change its potential
G protein-coupled receptor (GPCR): Contain seven transmembrane a helices and activate GTP-binding proteins
Receptor kinase (ex. receptor tyrosine kinase): Dimerize and activate their cytoplasmic protein-kinase domain to phosphorylate specific tyrosine residues of cytoplasmic substrate proteins
Nuclear receptor: function as ligand-regulated transcription factors
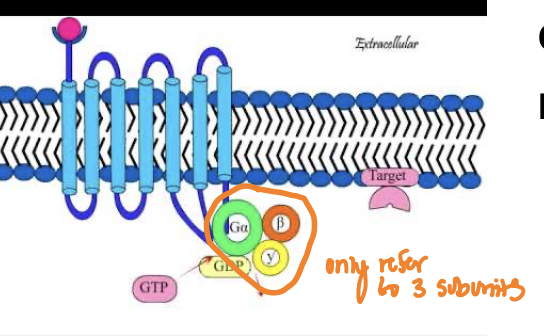
What’s the basic principle of G protein coupled receptors
G protein-coupled receptors (GPCRs) are a large family of membrane receptors that detect external signals and activate intracellular signaling pathways through the engagement of G proteins.
many different names but all involve G-protein
Found in all eukaryotes
They undergo conformational changes upon ligand binding, initiating signal transduction cascades that regulate various physiological processes.
Has 3 subunits: alpha (a), beta (B) and gamma (y)
The alpha subunit is a GTPase: Homologous to Ran and Rho and all the other GTPases
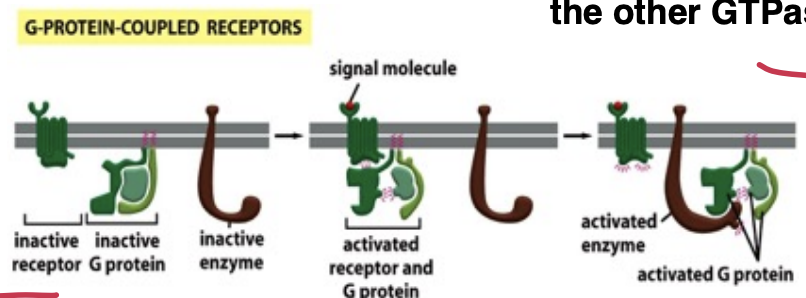
What’s the basic basic principles of signal transduction by GPCR
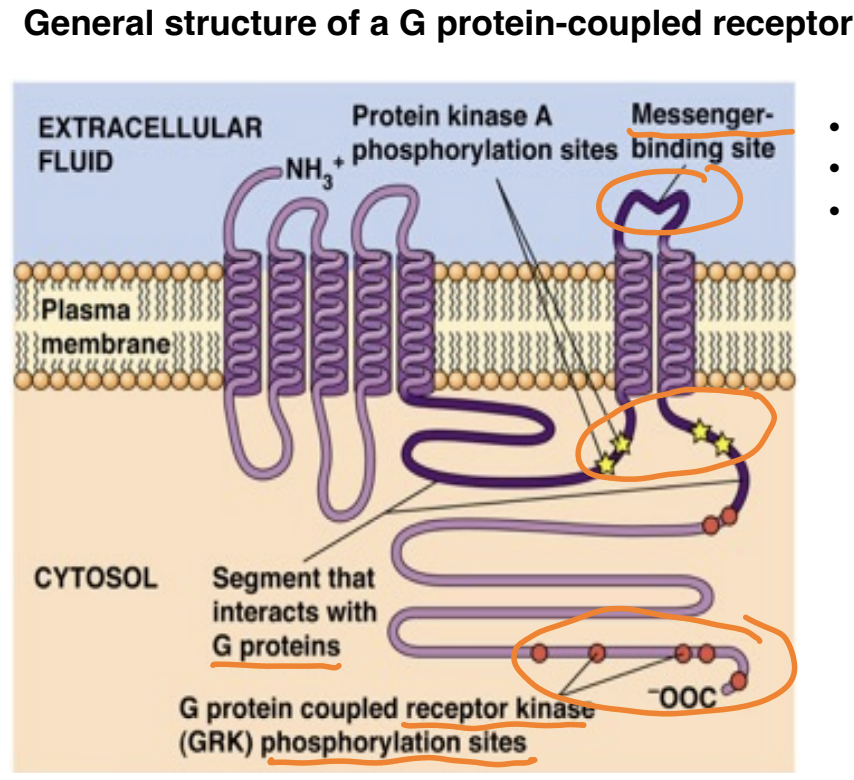
Downregulation = the decrease in receptor sensitivity, often due to prolonged stimulation, to prevent overstimulation of the signaling pathways.
GRK phosphorylation sites for receptor downregulation
PKA is activated by GPCR and also can particpate in downregulation
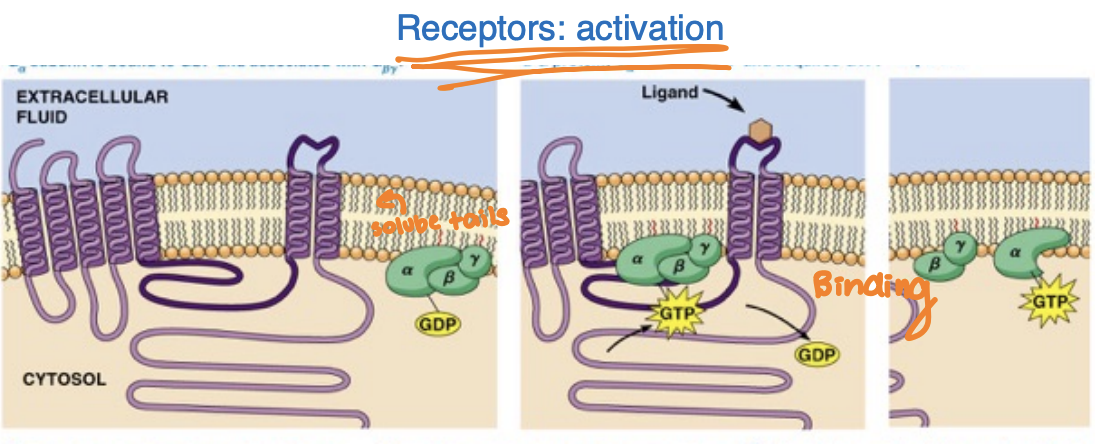
Signal transduction by GPCRs involves the recognition of ligands, leading to conformational changes that activate associated G proteins. This activation triggers downstream signaling pathways, resulting in various cellular responses.
Activation:
ligand binding to the receptor extracellular domain changes the conformation of its intracellular domain
receptor’s affinity for G proteins increases and the receptor binds the trimeric G protein
A GDP is exchanged for GTP on the Ga subunit which activates it and promotes association with the effector
A GEF (guanine nucleotide exchange factor) is the GPCR itself
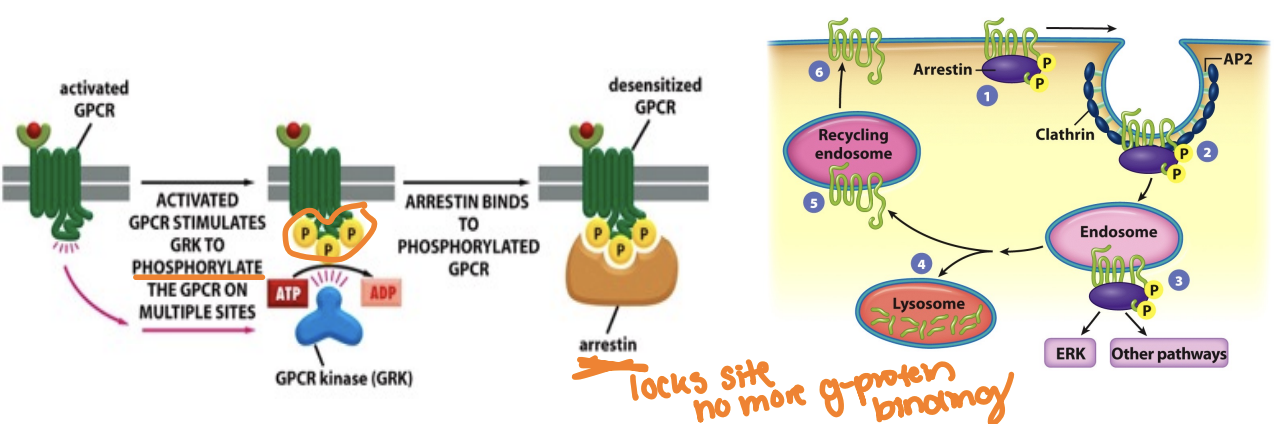
What’s the role of GRK and arrestin
Helps inactivation: termination of the response
must be turned off can't always be turned on
G protein-coupled receptor kinases (GRKs) phosphorylate active GPCRs, leading to their desensitization
desensitization: by blocking active site receptors from turning on additional G proteins (even though they are ligand bound)
Arrestins bind to these phosphorylated receptors, preventing further G protein activation and facilitating receptor internalization.
compete with G proteins to bind GPCRs
(if receptors are recycled and returned to the cell surface, the cells remain sensitive to the ligand and are said to be resensitized)
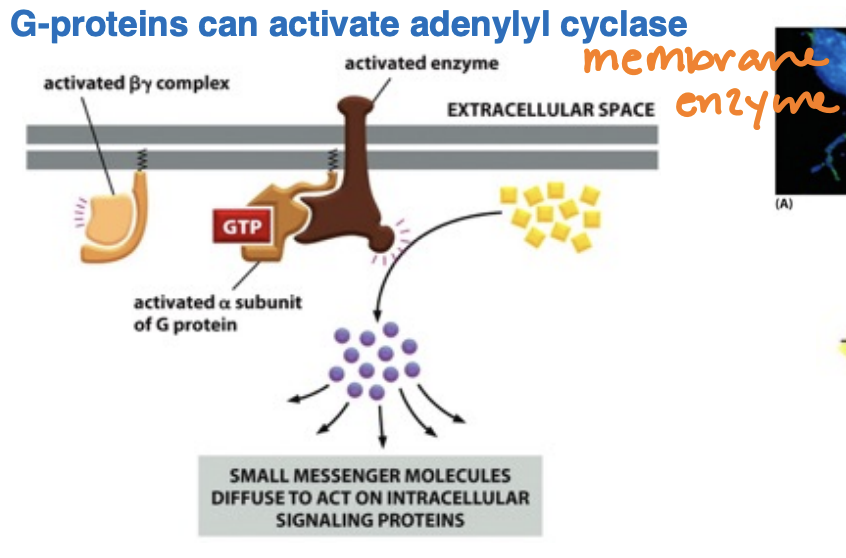
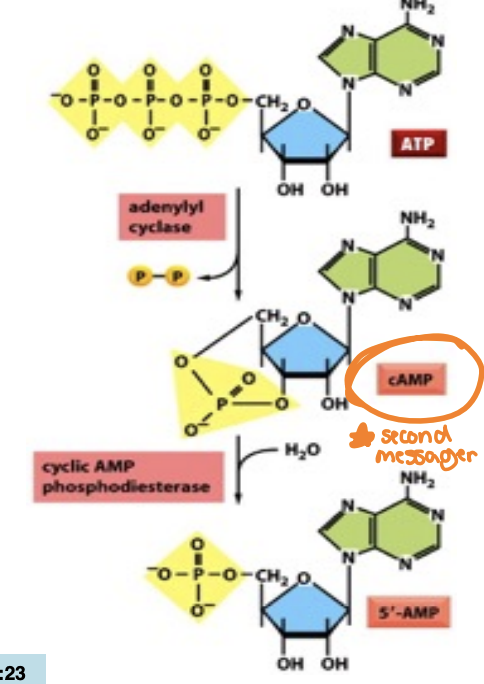
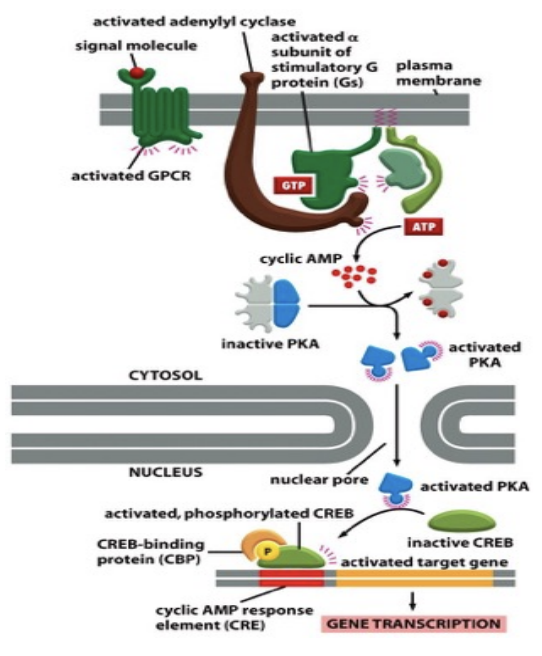
What’s the role and function of adenylyl cyclase and how cAMP is generated
Adenylyl cyclase is an enzyme that converts ATP to cyclic AMP (cAMP) in response to G protein activation
Adenylyl removes two phosphates as pyrophosphate
cAMP is shortlived (unstable) hence hyrdrozyled to 5’-AMP
serves as a second messenger, relaying signals from GPCRs to activate protein kinases, thus initiating various cellular responses.
Reaction is driven in the forward direction by the hyrdolysis of pyrophosphate
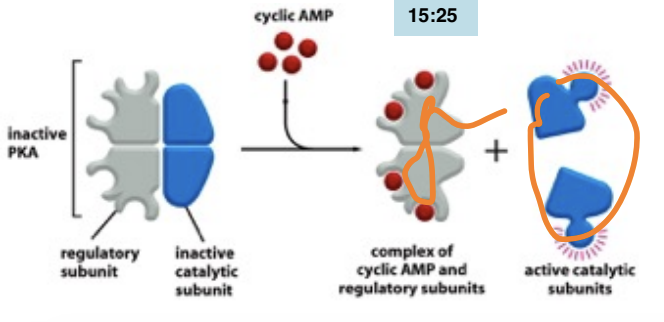
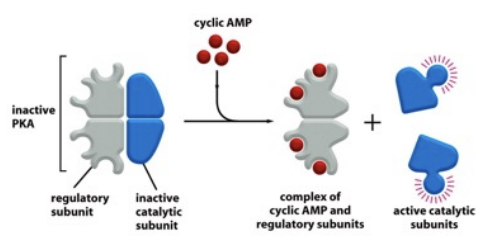
What’s the relationship between cAMP and PKA
Cyclic AMP (cAMP) activates protein kinase A (PKA) by binding to its regulatory subunits, leading to the release of its catalytic subunits and enabling phosphorylation of target proteins.
targets PKA (signals)
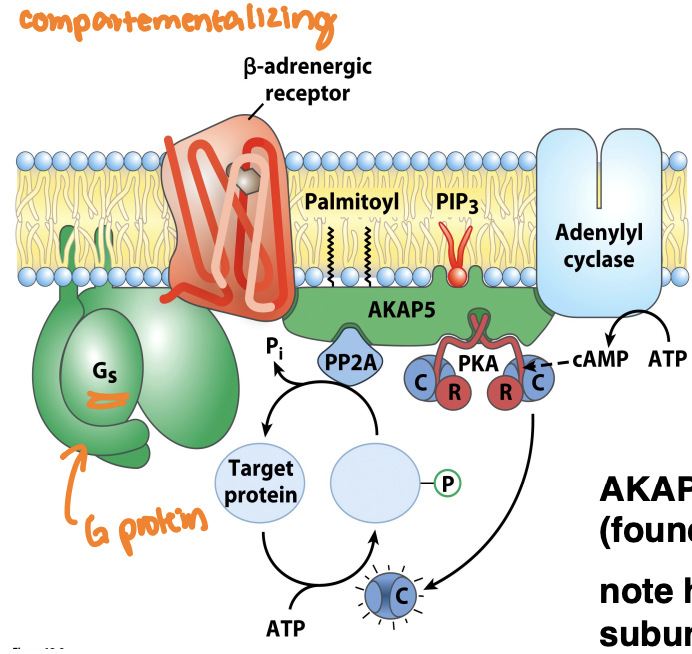
What’s the role of AKAP
A-kinase anchoring proteins (AKAPs) spatially organize the localization of protein kinase A (PKA) and other signaling proteins, thereby facilitating specific signaling pathways and enhancing the efficiency of cAMP signaling.
interacts with regulatory subunits of PKA by anchoring them in close proximity to their substrates so that pathways can efficiently transmit signals within the cell, ensuring rapid and localized responses to cAMP levels.
Lots of AKAPS (signaling hubs)
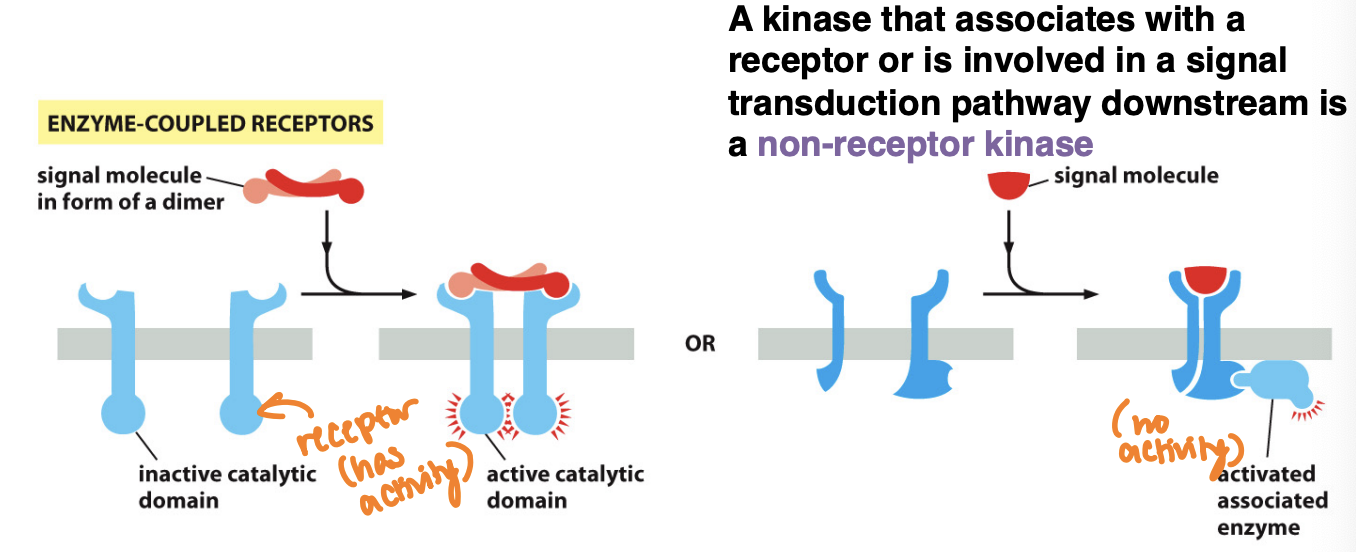
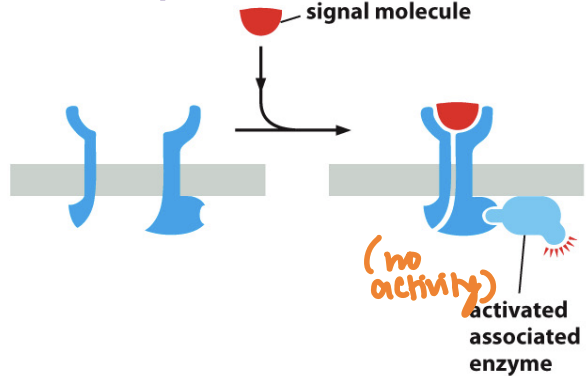
What’s an enzyme coupled receptor
A type of cell surface receptor that, upon activation by a ligand, activates an associated enzyme, triggering a cellular response
are transmembrane proteins that bind ligands
cytosolic domain either contains an intrinsic kinase activity (or associates directly with kinease
These receptors play a crucial role in various signaling pathways
Most common are RTKs
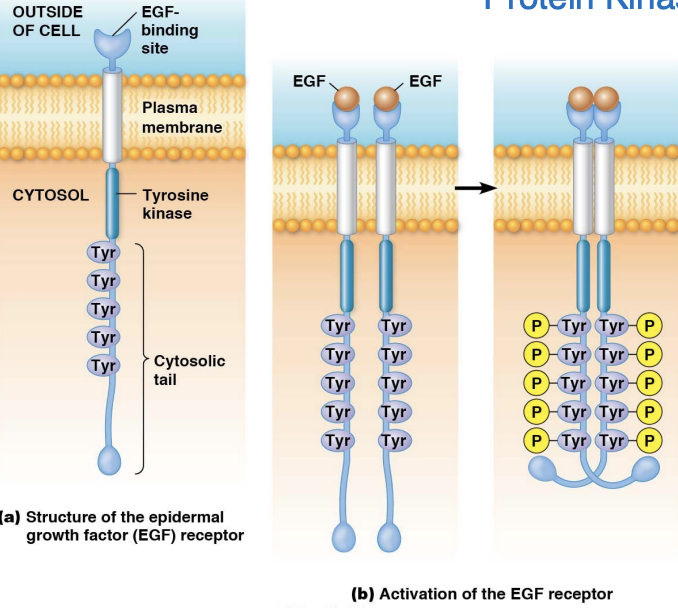
What’s a receptor tyrosine kinases (RTKS)
Receptor tyrosine kinases (RTKs) are a class of membrane receptors that, upon ligand binding, undergo dimerization and autophosphorylation, activating their intrinsic kinase activity and triggering downstream signaling pathways involved in cell growth, differentiation, and metabolism
Note: a tyrosine kinase receptor would be a receptor that had a tyrosine kinase as a ligand which is NOT what these receptors are – they themselves have tyrosine kinase activity and are therefore Receptor Tyrosine Kinases (RTKs)
What’s the characteristics of RTKs
Receptor tyrosine kinases (RTKs) typically possess an extracellular ligand-binding domain, a single transmembrane helix, and an intracellular kinase domain that can phosphorylate tyrosine residues on themselves and other proteins.
What is the non-receptor kinase
A type of kinase that is not associated with a membrane receptor but can phosphorylate target proteins in signaling pathways, often involved in cellular responses to various stimuli.
A kinase that associates with a receptor or is involved in a signal transduction pathway downstream is a non-receptor kinase
These kinases play a crucial role in intracellular signaling, activation of other proteins, and regulation of cell functions.
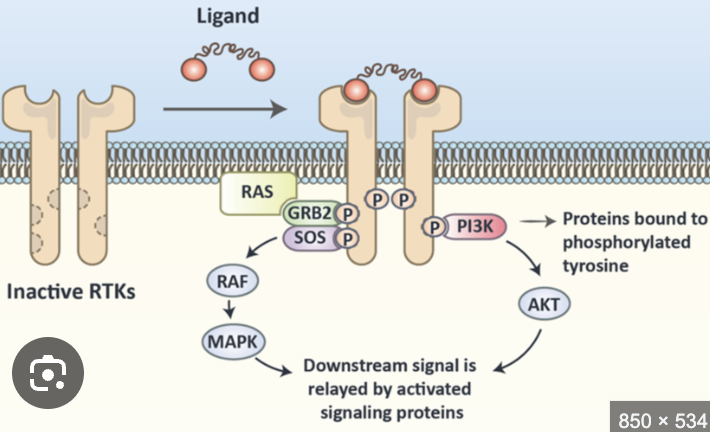
What is receptor dimerization (involves RTKs) + Autophosphorylation vs trans-autophosphorylation
Receptor dimerization happens when two receptor proteins (often on the cell surface) bind together (dimerize) after a signaling molecule (ligand), like a hormone or growth factor, attaches to them.
ligand itself can be a dimer
the receptors can be:
Identical (called homodimers)
Different but related (called heterodimers)
each monomer has both a tyrosine kinase activity AND individual tyrosines to be phosphorylated
a monomeric ligand can interact with two receptor monomers
Autophosphorylation = a receptor adds phosphate groups to itself on specific amino acids (usally tyrosines
trans-autophosphorylation = tyrosine kinase from one monomer phosphorylates the tyrosine residues on the other identical monomer
two monomeric ligands can bind independently to to receptor monomers promoting dimerization
Phosphorylated tyrosines on the receptor tails act as docking sites for other intracellular signaling proteins.
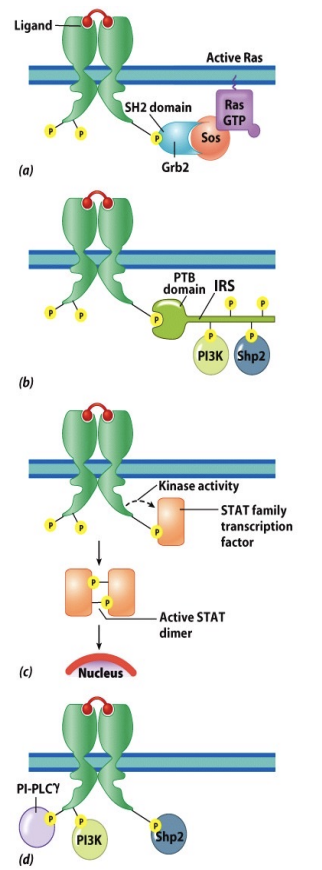
Activation of Downstream Signaling Pathways (DOMAINS)
SH2 and PTB domain proteins are:
Adaptor proteins that bind other proteins
Docking proteins that supply receptors with other tyrosine phosphorylation sites
Signaling enzymes (kinases) that lead to changes in cell
Transcription factors
Signal transduction by RTKs is usually terminated by internalization of the receptoprimarily through clathrin-mediated endocytosis
Some RTKs may bind to the clathrin adaptor protein AP-2, or may be targeted by ubiquitination by ubiquitin ligases through SH2 domains or adaptor proteins
Internalized RTKs can have several alternate fates; degraded in lysosomes or returned to the plasma membrane
What is SRC
SRC is a non-receptor tyrosine kinase (but still a type of tyrosine kinase) that is involved in signal transduction pathways
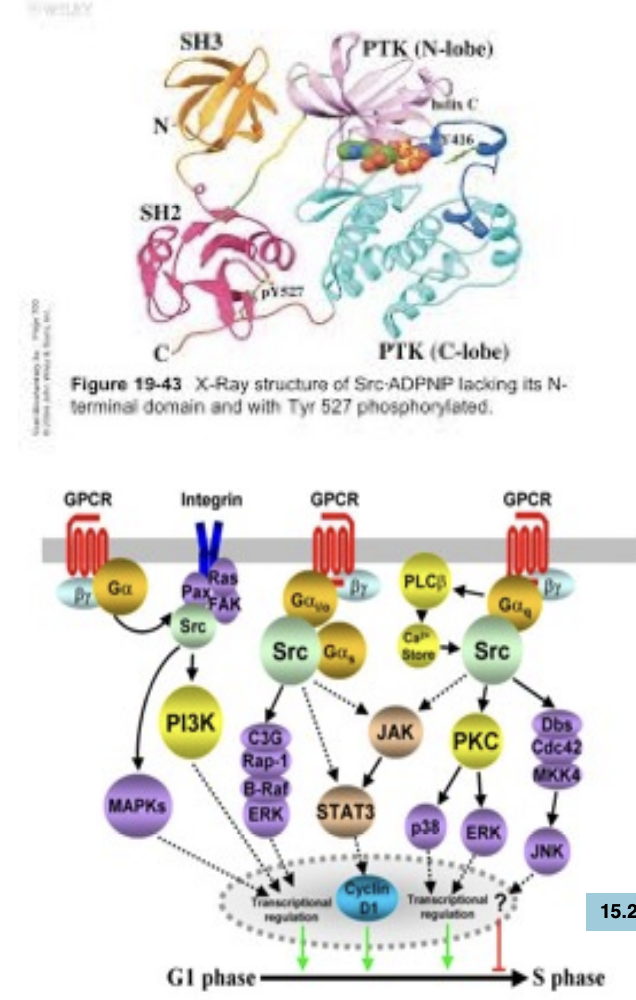
What’s the role of src homology domains and PTB domains
SRC homology (SH) domains and phosphotyrosine binding (PTB) domains are involved in recognizing and binding to phosphorylated tyrosine residues on target proteins, thereby mediating protein-protein interactions and facilitating signal transduction pathways.
SRC has 4 domains: 2 of these domains (SH2: src -homology 2 SH3: src -homology 3) are involved in binding to other proteins
these two domains turn out to be very common (conserved) in proteins involved in binding to other proteins in signal transduction
Terms to be familiar with: Oncogene, Proto-oncogene, Onco-protein, Proto-oncoprotein, Gain of function mutation
These are protein domains that facilitate protein-protein interactions, with src homology domains specifically recognizing phosphorylated tyrosine residues and PTB domains binding to phosphotyrosine-containing motifs.
oncogene (mutated to be always on)
proto-oncogene (normal gene)
oncoproteins (gain-of-function mutation)
proto-oncoproteins (normal protein)
Gain of function mutation = a mutation that leads to a protein being constitutively active or overexpressed, resulting in enhanced cellular functions or abnormal growth.
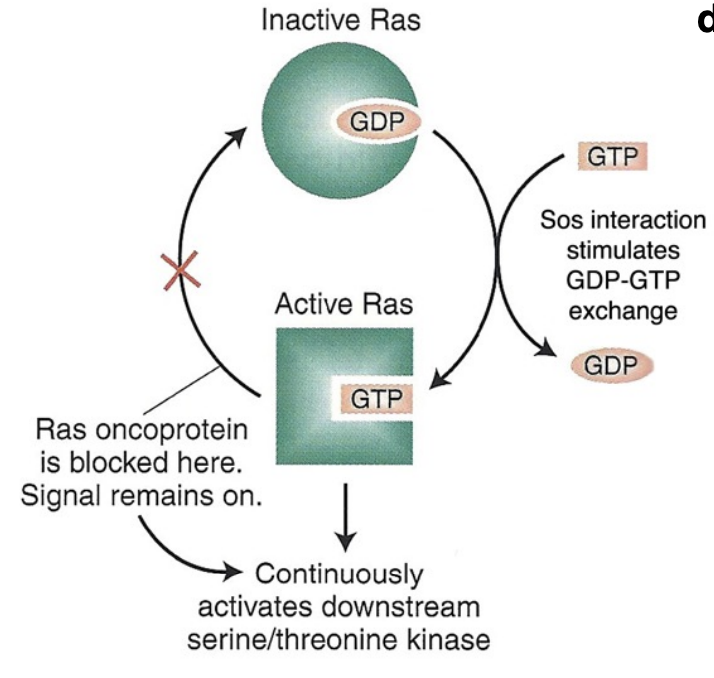
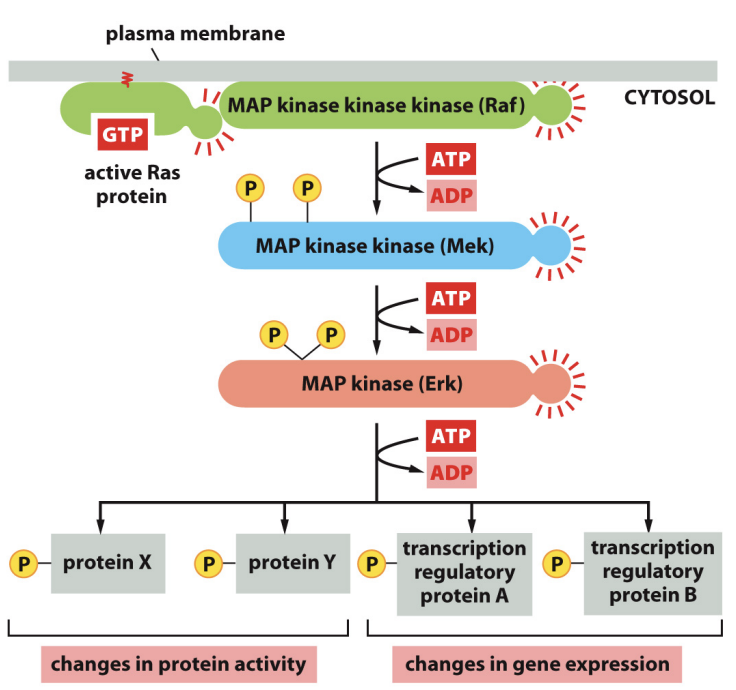
What’s the steps of the Ras-Map pathway beginning with ligand binding
The Ras-MAP pathway begins with ligand binding to a receptor tyrosine kinase, leading to receptor activation and autophosphorylation. This activates Ras, which then initiates a cascade involving MAP kinases that regulate cellular responses such as proliferation and differentiation.
This signal transduction system is highly conserved from yeast to humans
Ras is just associated (changes conformation)
by Ras recruits Raf to the plasma membrane and helps activate Raf
to MAP kinase kinase (MEK), then actives it and ultimately leads to the activation of MAP kinase (Erk), which phosphorylates various target proteins to elicit specific cellular responses
Constitutively-activated Ras is an Oncoprotein dervied from an oncogene
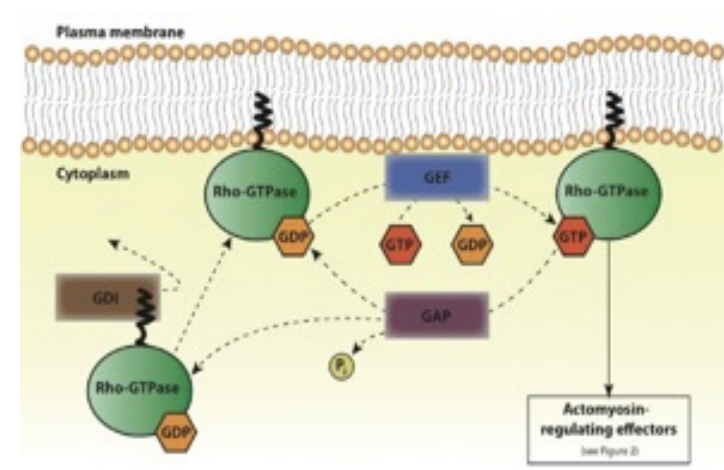
What’s the general role of Rho and what prevents it from binding to the membrane when inactive
Rho proteins (includes: Rho, Rac, CDC42) are GTPases that regulate actin and microtubules: controlling shape, motility, adhesion
they also help regulate: cell-cycle progression gene transcription membrane transport AND MORE
When inactive, Rho GTPases are bound to guanine nucleotide dissociation inhibitor (GDI) which prevents its binding to the membrane (via interactions with it’s GEF at the plasma) by maintaining it in a cytosolic state
Fun Historical facts that give perspective
SARC was the first oncogene discovered (it was in chickens)
Concept was then how was that translated into humans b/c viruses do not generally cause cancers in humans
Bottom line is that: Ras was the first human oncogene discovered, lots have been discovered since then