APChem
Chapter 1
Periodic Table Basics
- atomic number = number of protons
- molar mass (grams) of element
- horizontal = periods
- vertical = groups
- Group I = alkali metals
- Group II = Alkaline Earth Metals
- Group III - XII = Transition Metals
- Group VII = Halogens
- Group VIII = Noble Gases
- mass number = sum of neutrons and protons
- isotopes = elements (obviously same # of protons) varying neutrons
Moles
- Avogadro’s # 6.022 * 10^23 particles per mole
- M = molarity or mol/L (* remember mol is moles)
- moles = grams/molar mass
- moles = PV/RT
- P = pressure (atm)
- volume (L)
- temperature K)
- R = gas constant 0.0821 (L atm / mol K)
- many gas occur at STP (standard temperature pressure), P = 1 atm and T = 273 K
- can convert directly between volume of gas and number of moles
- moles = liters/ (22.4 L/mol)
Molarity
- M = moles of solute / liters of solution
Percent Composition
- To calculate you must separate the compound by elements
- Take the molar mass of each and find the mass of each element in compound
- convert by dividing each mass of substance by the TOTAL mass of ENTIRE compound and multiply by 100
Empirical & Molecular Formulas
- Finding empirical formula of compound
- start by assuming the percentages total up to 100g (ex: 55.6% is 55.6 g or 7.11% is 7.11g)
- Divide each gram you get by the molar mass of the pure element
- Divide all the moles now by the lowest number of moles calculated above
- Should receive the subscript of the elements in formula
- Find the molecular formula from empirical formula
- Find mass each element
- Use molar mass of element x subscript
- Combine to find total molar mass of compound
- divide mass given by molar mass of compound and find molecular formula
Coulomb’s Law concepts
- F(columbic) = magnitude of positive charge x magnitude of negative charge / distance btwn negative charge
Bohr’s Model
- electromagnetic radiation: electrons jump to higher energy level
Photoelectron spectroscopy
- ionization energy: amt of energy necessary for electrons to be ejected
- kinetic energy: energy of incoming radiation must be conserved & any of energy that does not go into breaking electron free from nucleus is converted in KE
- EQUATION: incoming radiation energy = binding energy + kinetic energy (ejected electrons)
Spectra
- photoelectron spectrum (PES): amt of ionization energy for all electrons ejected from nucleus
- subshells: shape of space electron can be found
Electron Configuration
- s subshell: max 2
- p subshell: max 6
- d subshell: max 10
- f subshell: max 14
- Shorthand or normal notation (remember brackets for shorthand)
Aufbau principle: building up electron config of atom, electrons placed in orbitals, subshells, and shells in order of increasing energy
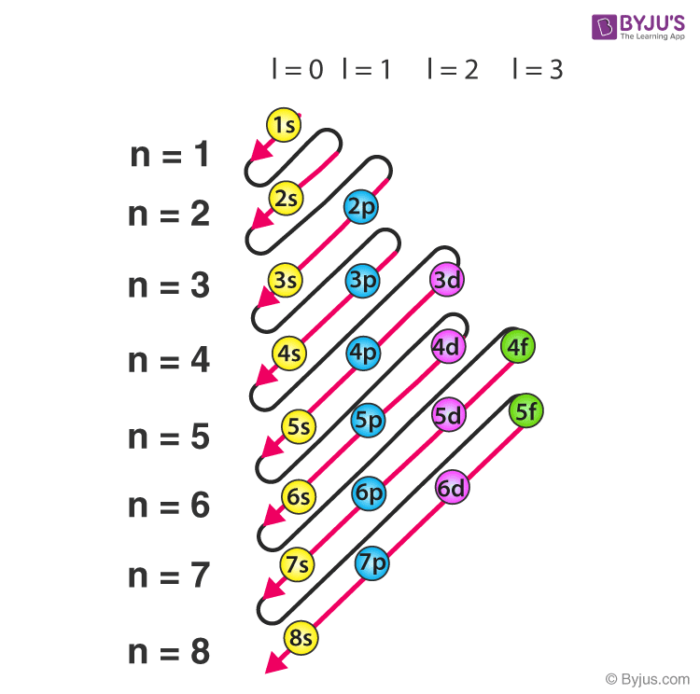
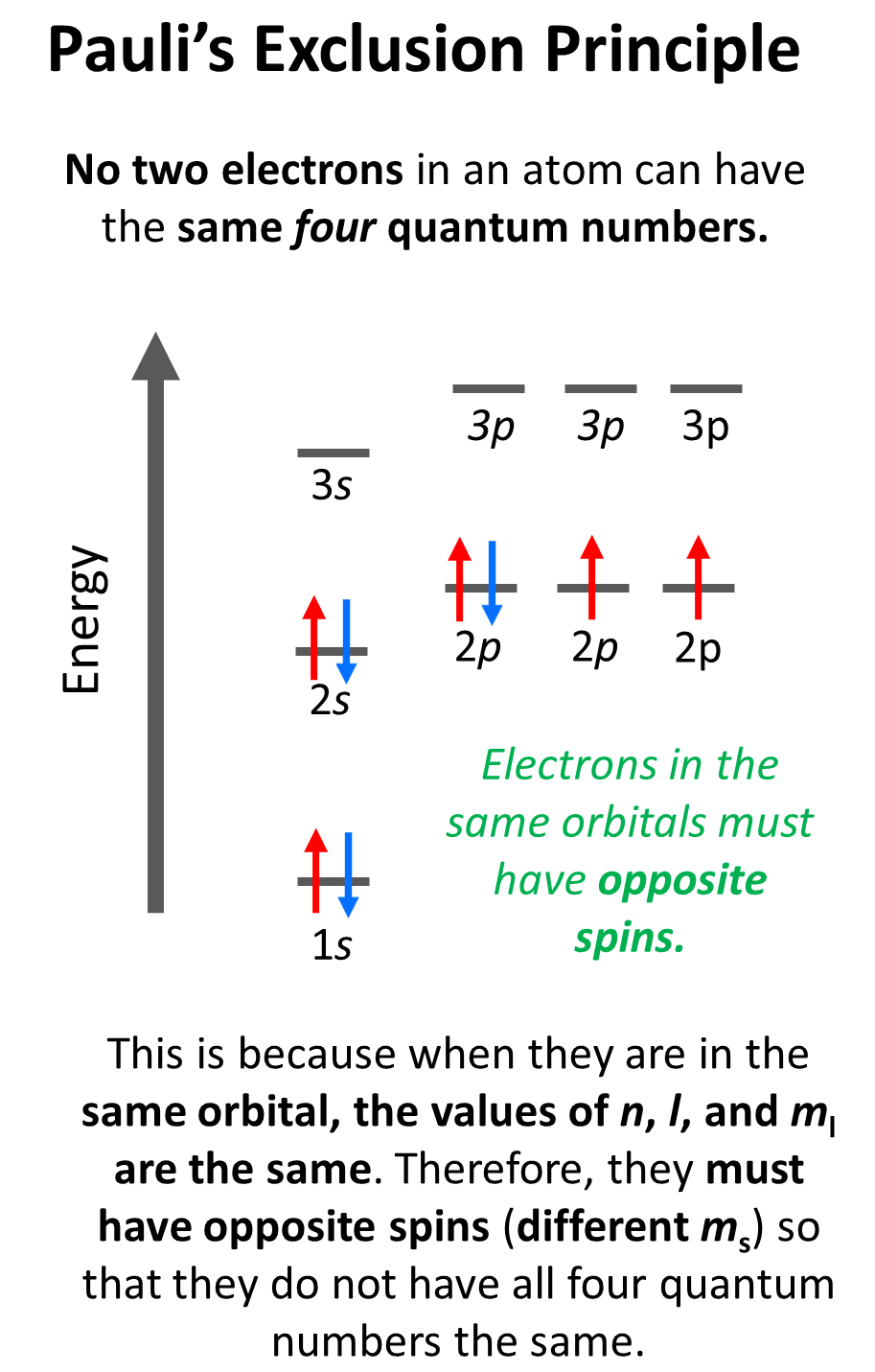
Pauli Exclusion Principle: two electrons share orbital cannot have same spin * when draw arrows make sure they are opposite
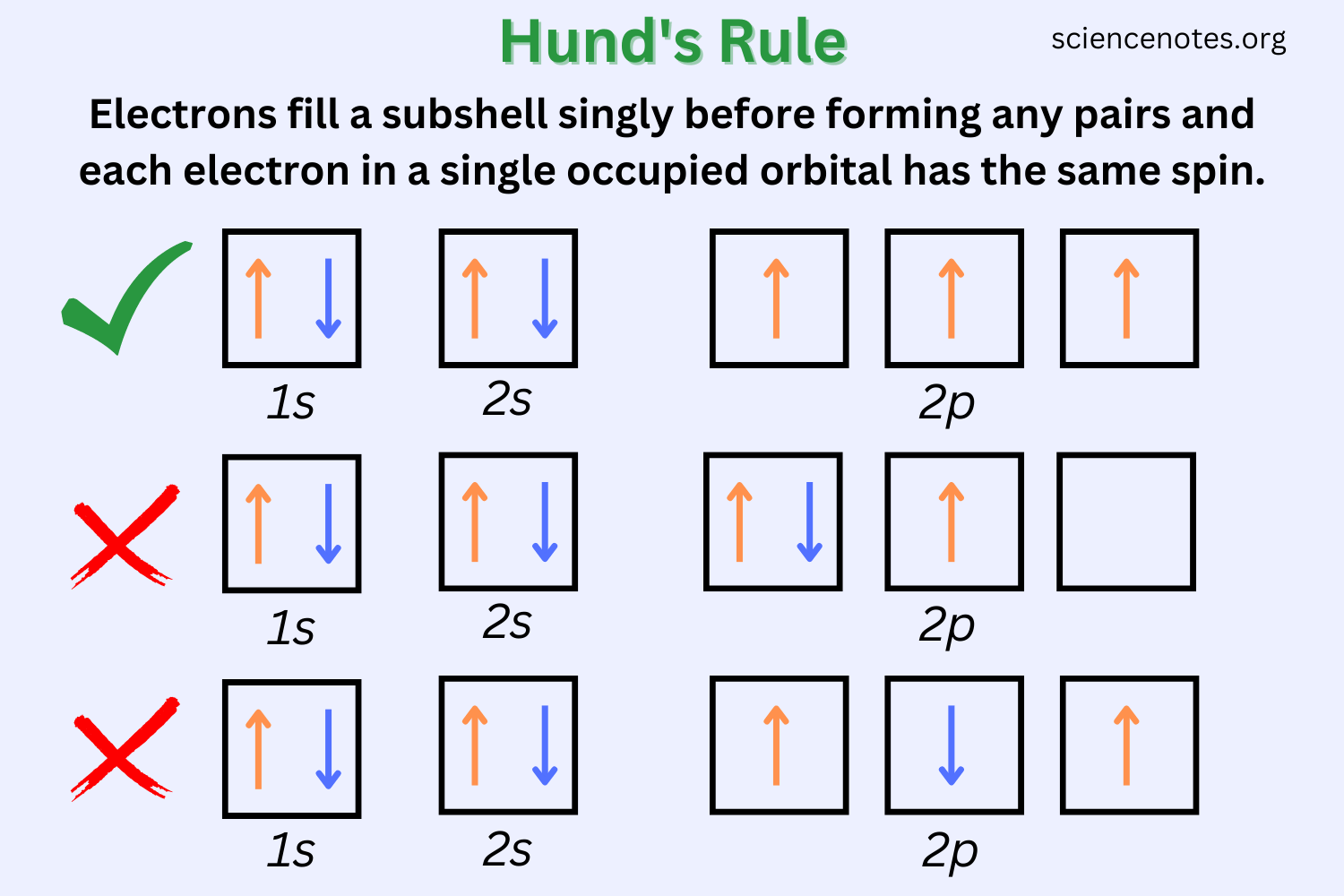
Hund’s Rule: when electron is added to subshell, always occupy empty orbital if one is available
Predicting Ionic Change:
- valence electrons in outermost s and p subshells
- ion: atom either gained or lost electrons
- cation (positive charge) anion (negative charge)
Periodic Trends
- electrons are attracted to protons in nucleus of atom
- closer electron is to nucleus, more strongly it is attracted
- more protons in nucleus, more strongly electron is attracted
- electrons repelled by other electrons in atom; electrons are between valence and nucleus, valence electron will be less attracted to nucleus (electron shielding)
- completed shells are “stable”; atoms will add or subtract valence electrons to create 'complete’ shells if possible
Atomic Radius
- approximate distance form nucleus of atom to its valence electrons
- LEFT TO RIGHT OF PERODIC TABLE
- protons are added to nucleus → valence electrons more strongly attracted → decreases atom radius
- IF electrons added in same shell, NOT much shielding effect since abt same distance from nucleus
- TOP TO BOTTOM OF PERODIC TABLE
- \