Chemistry - Atomic Structure
1/12
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Nucleus
the center of the atom
composed of protons and neutrons
Protons
found in the nucleus
determines the identity of an atom/what element an atom is
heavy in mass (about 1 amu)
positively charged (+1)
Atomic Number
the number of protons
identifies an element
all atoms of the same element will have the same atomic number
no two elements have the same atomic number
Neutrons
found in the nucleus
neutral charge (0)
heavy in mass (about 1 amu)
holds the protons together in the nucleus — because the protons of an atom all have a positive charge, they would repel from one another without the neutrons
Electrons
located outside the nucleus
in constant motion — gives an atom that “fuzzy edge”
negatively charged (-1)
virtually no mass; smallest of the 3 subatomic particles
responsible for many of the properties of individual atoms and are involved in all chemical reactions
Neutral Atoms
contains the same number of electrons as protons
have a neutral charge (0)
Mass Number
the total number of protons and neutrons in an atom
most of the mass depends on the nucleons
Number of Neutrons
rarely obvious
will have to do a math problem to find the number of neutrons;
Mass Number - Atomic Number = Number of Neutrons
not given a specific mass number, use the periodic table’s average atomic mass and round to the nearest whole number
given a specific mass number, use that as your mass; not the periodic table
Isotopes
atoms of the same element with different numbers of neutrons
only way to tell them apart is by their masses
Nuclear Reaction
difference in mass/amount of neutrons is important to consider
examples are radioactive decay and fission
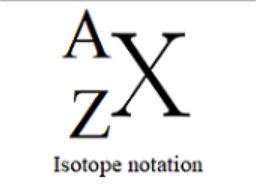
Isotope Symbols
X = element symbol
A = Mass Number
Z = Atomic Number
Isotope Names
another way to refer to isotopes
simply add the mass number to the name of the element
example: boron-11
Average Atomic Mass
shown on each square of the periodic table
equation: %/100 * amu + %/100 * amu