BIOL 2022 - Introduction to the immune system and innate immunity
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
role of the immune system
defence against infectious disease
complex to deal with a range of organisms
protection against some tumors
vaccines offer new hopes for infection and cancer therapy
evolves very fast
needs to be regulated —> chronic immune response can cause disease
E,g: sepsis, autoimmunity, type 2 diabetes
has 2 arms: the innate and the adaptive immune system
cells of the immune system
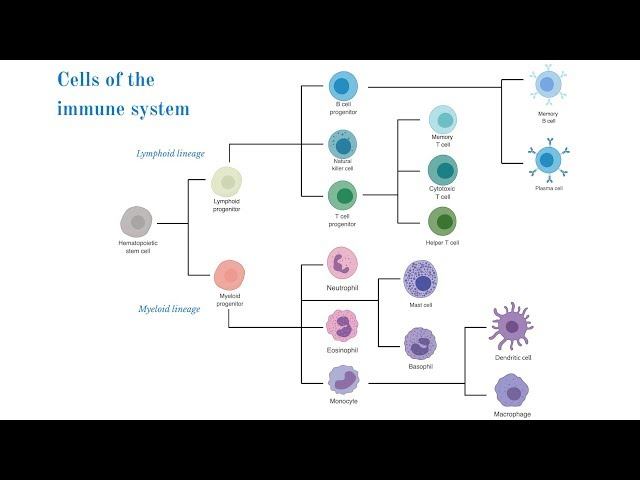
HEAMOPOETIC STEM CELL
give rise to the cells of the immune system
either lymphoid or myeloid lineage
generated in the bone marrow
CYTOTOXIC T CELLS
occur after the generation of active t cells
interact with the antigen on surface of cells which signal that the cell is infected
drives downstream signalling which causes the immune attack on infected cells
MACROPHAGES
can recognise, digest, take up pathogens and present their antigens
B CELLS
also recruited by dendritic cells but their role is to produce antibodies
COMPLEMENT
not a cell but binds to pathogens which leads to direct lysis
produced in the liver and excreted in the blood
produced in response to threat
PART 1: INNATE IMMUNE SYSTEM
innate immune system
quick and non specific response
results in the release of chemical mediators which create inflammation which supports the immune response
can lead to the direct removal of the infectious agent
have receptors which recognise the pathogen and can distinguish between viruses and bacteria
activation drives downstream signalling to produce mediators (E.g: cytokines and interferons)
made up of the humoral and cellular immune system
HUMORAL: driven by cytokines, proteins, mediators
CELLULAR: phagocytes and NK cells involved in taking up or killing the cells (cellular components)
the innate immune system is in control of detection of pathogens and the immediate response to them
it can then recruit cells from the blood and send signals to promote the required response
in order to activate the innate immune system it must breach one of the bodys natural barriers
bodys natural barriers
SKIN
anatomical mediator
very protective barrier
PHYSIOLOGICAL BARRIERS
includes:
temperature
pH
chemical mediators
INFLAMMATORY BARRIERS
leakage of serum proteins with antibacterial activity
influx of phagocytic cells into the affected area
signs of inflammation
swelling (tumor)
redness (rubor)
pain (dolor)
heat (calor)
and it was later added: functio laesia —> loss of function
when a barrier is peirced
histamine is produced as a response to the skin being peirced
dilation and increased leakiness of blood vessels due to the leakiness of tight junctions
phagocytes migrate to the area and consume the bacteria and cell debris so the tissue heals
toll like receptors
DISCOVERY
1995-1997
believed that the immune system needed a danger system to be activated
just prescence of foreign antibodies is not enough
receptors on immune cells must respond to danger
toll gene important in embryogenesis but later a toll knockout resulted in flies covered in fungus which proves that the toll gene has a role in immune defence
a group of genes important in immune sensing are the toll like receptor genes
the first proof of toll like receptors sensing microbial patterns was in toll like receptor 4
mutation in Lps gene will selectively impede liposaccharide signal transduction and results in resistance to endotoxin as TLR4 proteins role is recognition of LPS
pathogen recognition receptors
theres at least 12 types
has a structure which recognises PAMPs
LPS PAMP from gram negative bacteria initiates various innate responses
phagocytosis of pathogens
chemokines and cytokines to recruit cells
lysis of pathogens
there are a whole range of pathogen recognition receptors but toll like receptors are a subclass
toll like receptors (TLR4)
trigger signal cascades
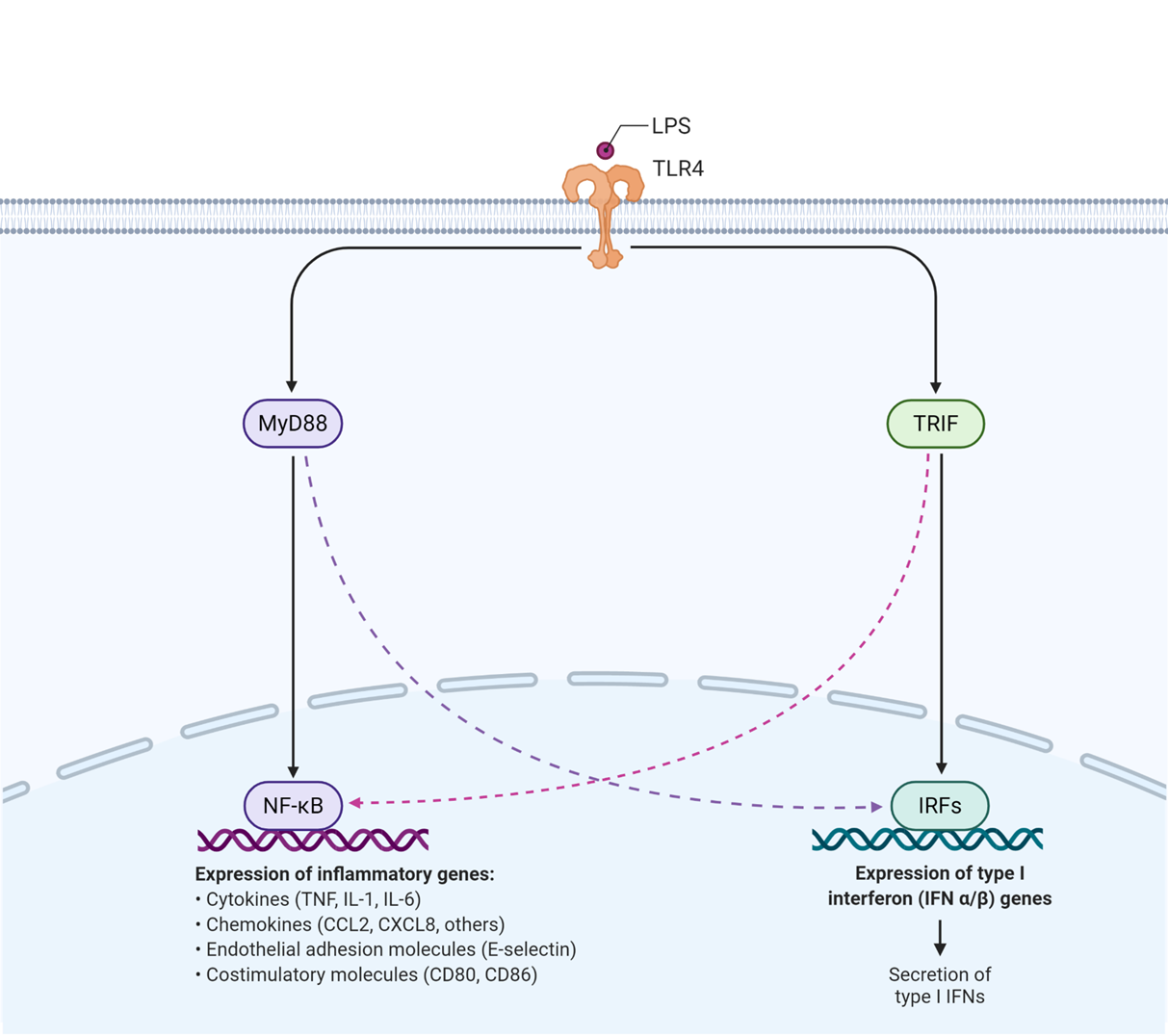
LPS interacts with toll like receptor 4 which causes a conformational change in the intracellular domain and can now interact with proteins
one of the proteins that it interacts with is MyD88 which is an adaptor protein that leads to upregulation of:
cytokines
chemokines
endothelial adhesion molecules whih allow glucosides to bind to and allow leukocytes to move into cells from blood
MyD88 also activates the costimulatory molecules which promote a t cell response
toll like receptor 4 also activates the proetin TRIF which activates
interferon expression through TFs
(interferons are important in viral recognition but also prepare cells to be recognised by immune cells)
how does signalling of toll like receptors work
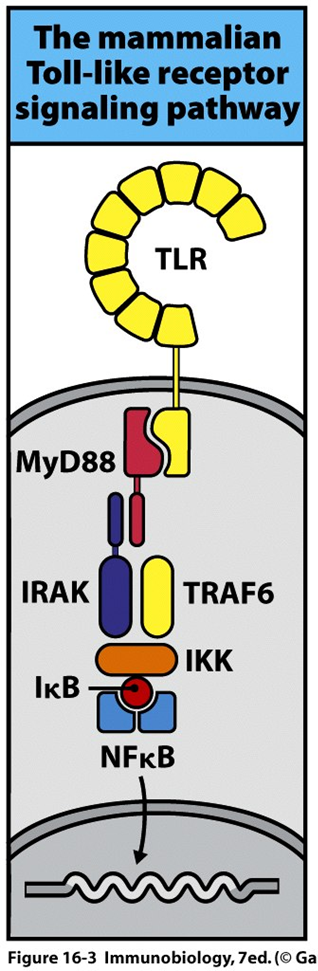
their extracellular structures are leucine rich structure which interacts with LPS of a range of gram negative bacteria
the intracellular region can then interact with MyD88 only when it undergoes conformational change
MyD88 then interacts with a range of kinases (IRAK 1 and 4) which phosphorylate the complex
phosphorylation generates a docking signal for TRAF6 which forms a dimer —> dissociates from the 1st part of the signalling cascade
this dimer then complexes with TAK1 which causes kinase activation
the IKK complex is phosphorylated and Ikappa b degraded.
this frees up and activated NFkappaB
activation of NFkappa B which is important for MAPK pathways —> causes transcription of TNFa and IL6
NFkappa B
in most mammalian cells, usually present in the cytoplasm associated with I kappa B
teh TAK1 is able to phosphorylate I kappa B causing dissociation
it then becomes ubiquitinated and degraded in the proteosome
NFkappa B can now move into the nucleus and initiate transcription of cytokines
eventually NFkappa B will move into the nucleus where it will reassociate ith Ikappa B
toll like receptors on macrophage/dendritic cells
both have toll like receptors but respond in different ways
both have external TLRs on the CSM which are useful for fungi parasites and bacteria
has internal TLRs in endosomes which can detect viruses
MACROPHAGES:
gene transcription from the activation of TLRs causes cytokine synthesis and oxidative burst
OXIDATIVE BURST:
increased oxygen radicals
various TLRs and various cytokines to differentiate between virus and bacteria
DENDRITIC CELLS:
the outcome is different
still results in cytokine production but also results in production of costimulatory molecules which is key for efficient antigen presentation
helps to activate t cells
various TLRs and various cytokines to differentiate between virus and bacteria
types of TLRs
TLR4 is the only TLR which responds to the TRIF pathway
TLR 1, 2, 5, 6 also can respond to PAMPs but they might be gram negative or bacteria with flagella
extracellular TLRs dont use the interferon pathway, they use the MyD88 pathway
endosomal receptors interact with ss or ds RNA from the viruses
TLR 3 is the only TLR which uses the interferon from TRF pathway and recognises ds RNA
cell types
NK CELLS
not technically part of the innate immune system but important for immune surveilance
DENDRITIC CELLS
produce a number of cytokines which allows other cells to be activated and drive specific immune response themselves
MACROPHAGES
phagocytosis and cytokine production
NEUTROPHIL
can phagocytose and produce potent enzymes
cytokines and interferons
cytokines are local mediators but can also act systemically if secreted in the blood
they also have their own receptors which act through the jaks and stats signalling pathway
LOCAL FUNCTION
they can signal to cells to put up barriers —> increased expression of TLRs
this might result in direct cell death
will recruit WBCs to the site of infection
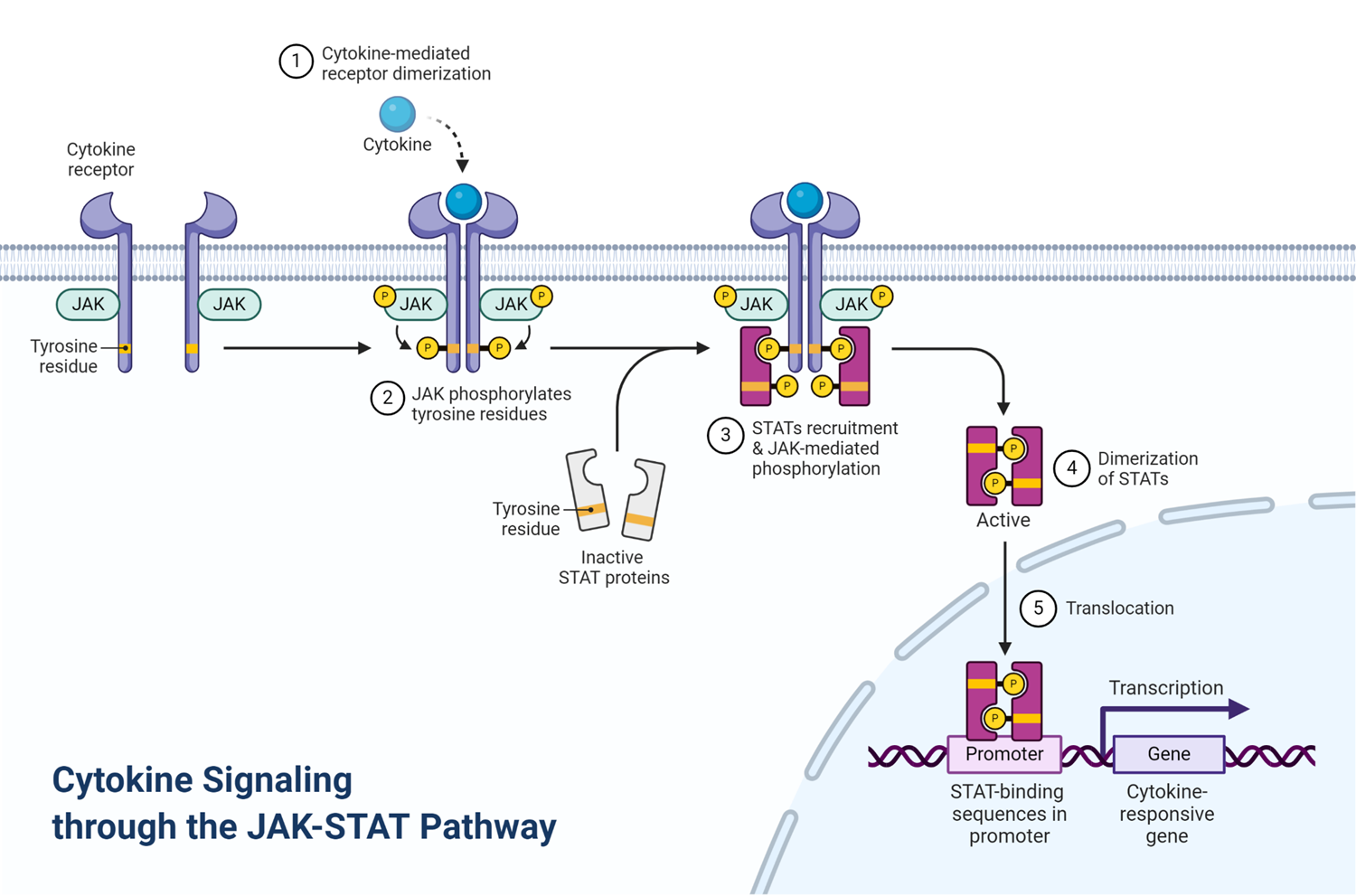
JAKS AND STATS
the janus kinase has 2 faces: one which interacts with the receptor, the other that initiates downstream signalling pathway
the janus kinase binds to receptor and phosphorylation of the tyrosine kinase creates a binding domain for SH2 domain of stats (signal transduction and activation of transcription)
stats activates a TF that binds DNA sequence specifically and promotes transcription from GAS element in response to cytokine stimulation
a combination of different jaks and stats allows immune cells to respond differently to different cytokines
not just the cytokine itself that signals danger but also the response to the cytokine
local vs systemic effects of cytokines
LOCAL
increased permeability
increased adhesion molecules
decreased flow rate
increased chemokine expression
increased activation degranulation
all of which leads to increased recruitment of immune cells
SYSTEMIC
acute phase proteins
hypothalamus: fever which acts as phsiological defence
mobilises cells from the bone marrow
interleukins
IL 1beta
activates vascular endothelium
produced by macrophages
promotes local tissue destruction
increased access of effector cells
IL 6
activates B cells
IL 8
recruits neutrophils
chemokines
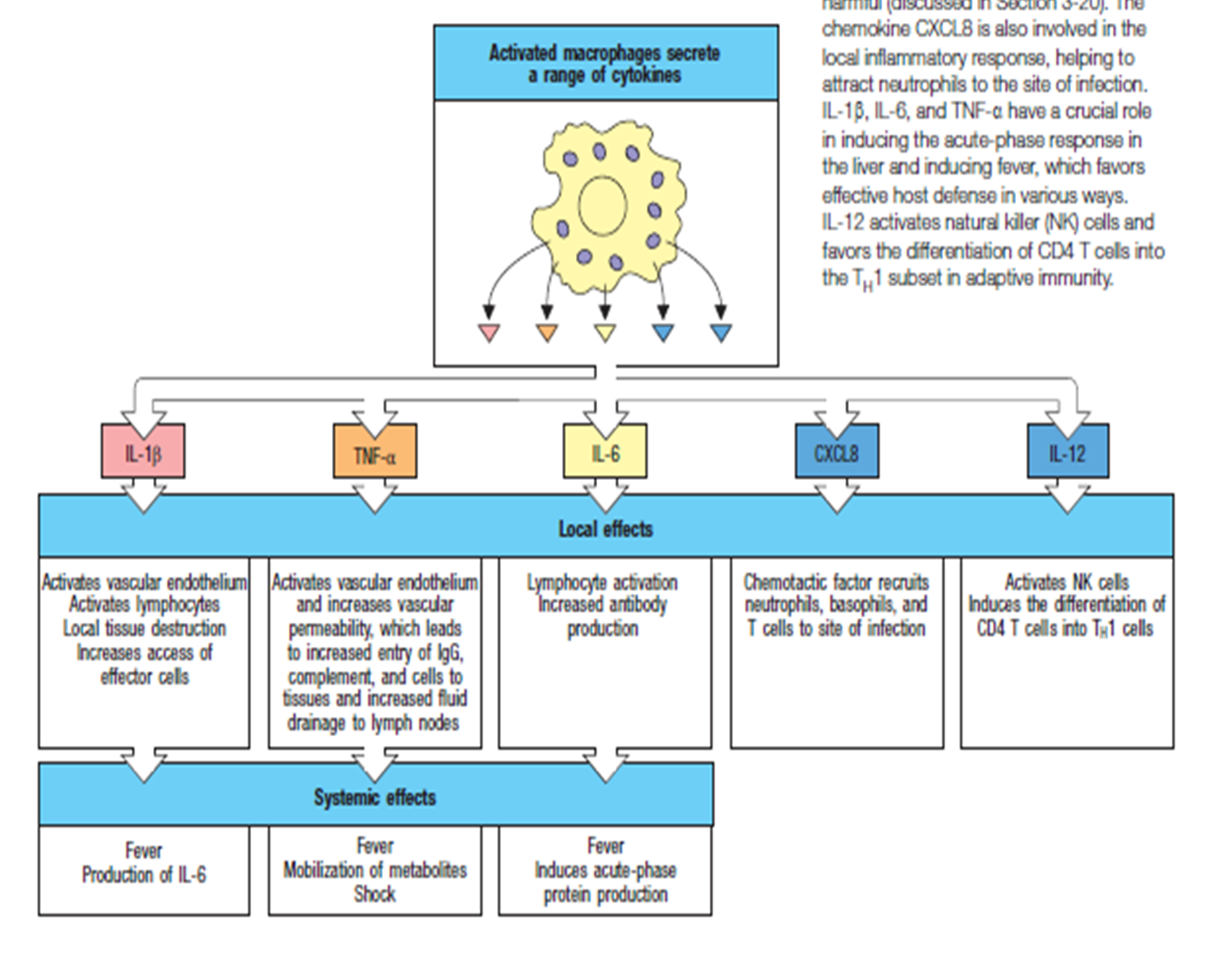
TNF alpha
causes complement activation
increased permeability
generates fever
cells needed for viruses
not recognised well by the innate immune system
macrophage needs to produce cytokines which activate T cells
IL12 is a good at this - it differentiates TH1 cells which produce LT and interferon gamma
IL4, 4, 6, 10, 13 differentiate TH2 cells to B cells
complement activation
complement system important for immediate response
hemolysis of red blood cells is mediated by antibody involved complement
has roles in innate and teh adaptive immune system
not only activated by antibodies, can be activated by other parts of innate IS
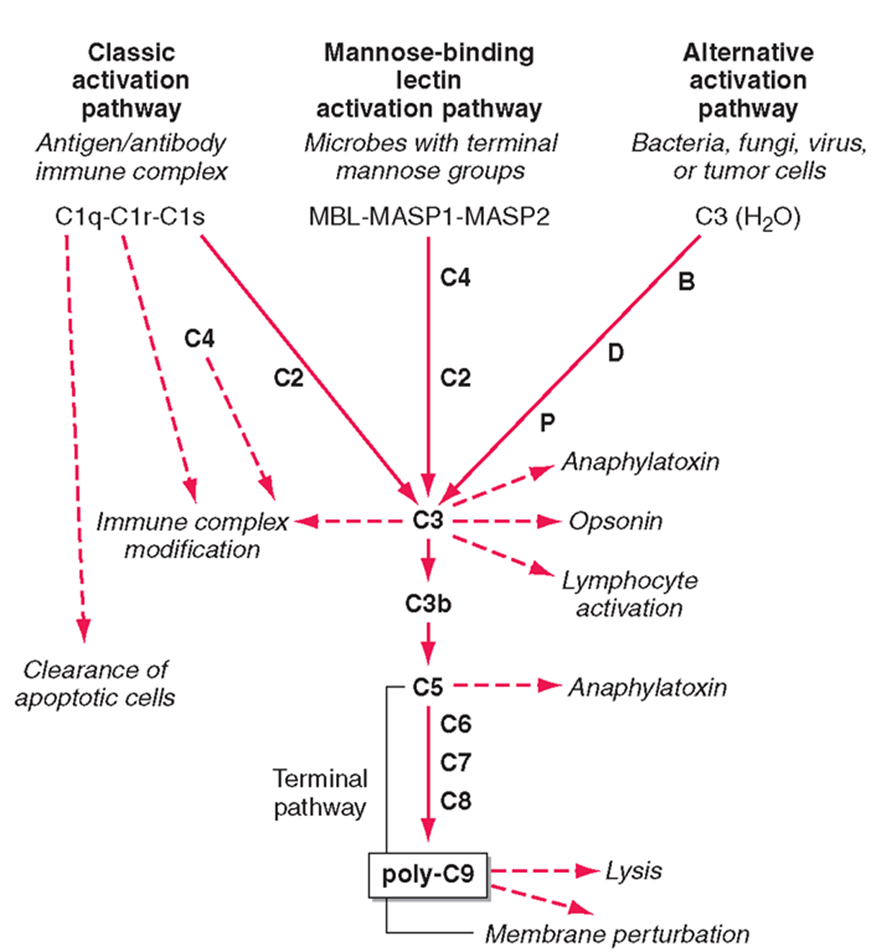
mammalian complement has 3 pathways:
classic activation
mannose binding lectin
alternative actiavtion
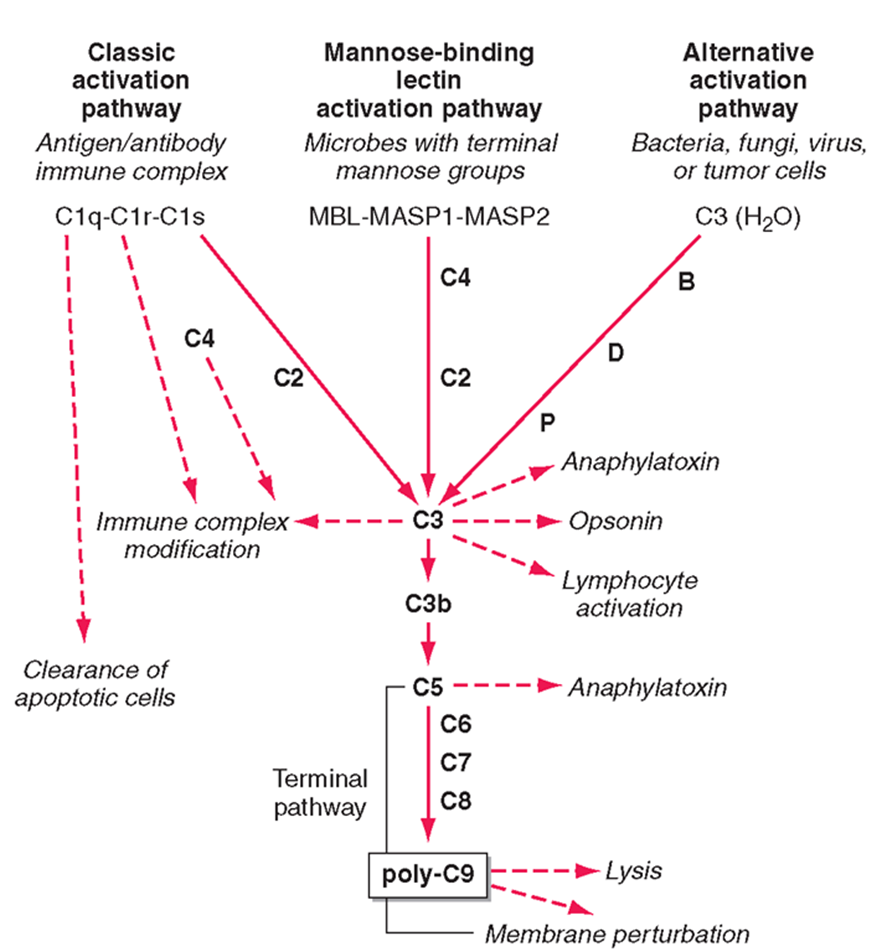
alternative activation
activation pathway used by pathogens
C3 in the blood is usually active, it undergoes small ammounts of spontaneous hydrolysis in water
in the prescence of the pathogen hydrolysis increases and can interact with B, D, P factors
interaction with these factors allows it to undergo a conformational change and be cleaved
the C3 protein is the central protein of all 3 pathways
theC3 confetase acts as an enzyme to cleave C3 protein in the blood (unhydrolysed)
cleaved C3 forms C3a, C3b and lymphocyte activation
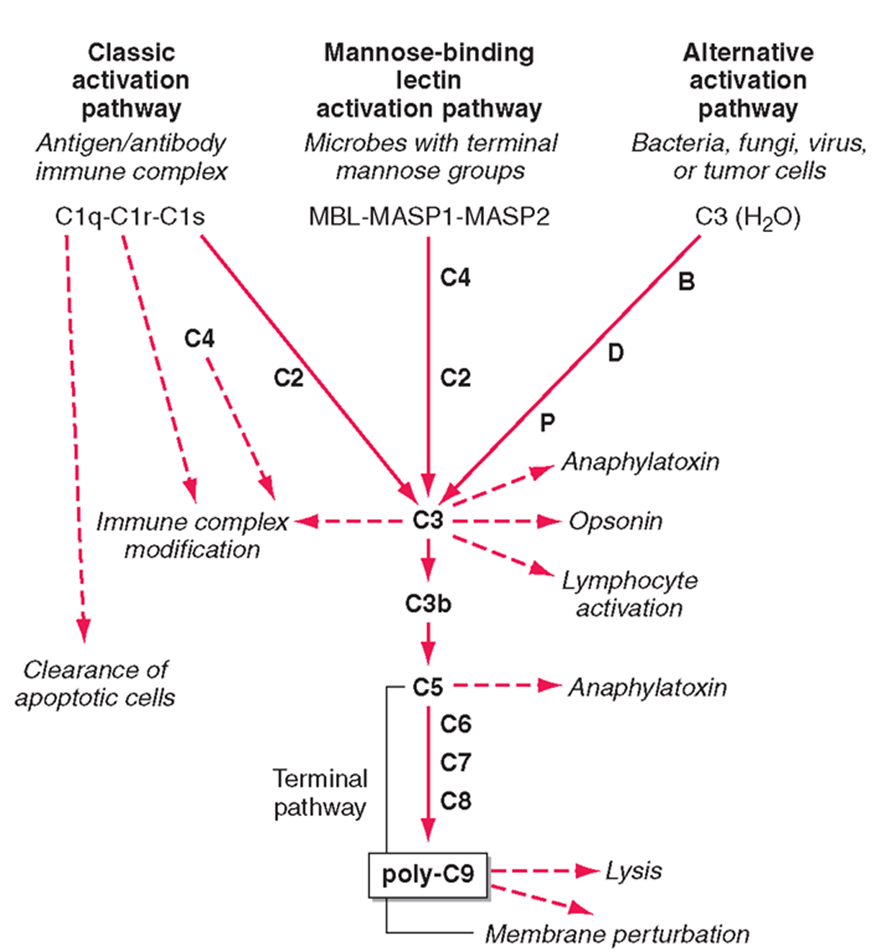
downstream effects of C3
ANAPHLATOXINS (C3a)
highly attractive for immune cells
OPSINS (C3b)
can bind and incorporate into cell membranes
forms a conformational change forming C5 confetase
C5 gets cleaved into C5a (an anaphylatoxin)
C5 can also bind to C6, C7, C8 to form poly C9 - a membrane attack complex which forms a pore in the membrane of bacteria and leads to the lysis of the membrane
mannose binding lectin pathway
complement pathway activates by sugar proteins, rather than structures on the bacteria
mannose sugars activates MASP1 and MASP2 which recruits C4 and causes C2 binding
this complex forms the C3 conferase which cleaves the central protein
C3a and C3b pathway then the same
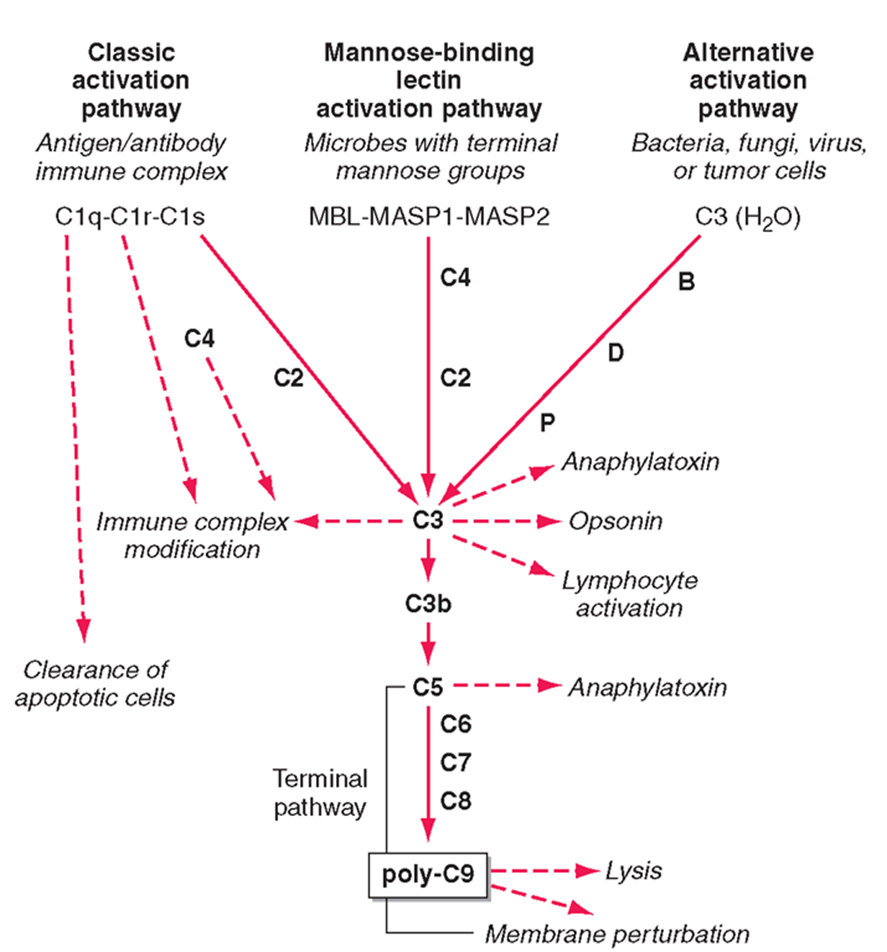
classical pathway
mediated by antibodies
antobodies bound to the membrane acts as a platform for Clq binding
Clq looks like 6 tulips and interacts with the fc compartment of an antibody
a small conformational change which allows binding of Clr and Cls to be cleaved
C4 and C2 can then bind to the antibody Clq complex and converted to C3 confetase
follows the same C3a and C3b pathway
key role of complement
produces opsonins
produces anaphylatoxins
direct killing of organisms
enhance antigen specific immune response
maintain homeostasis
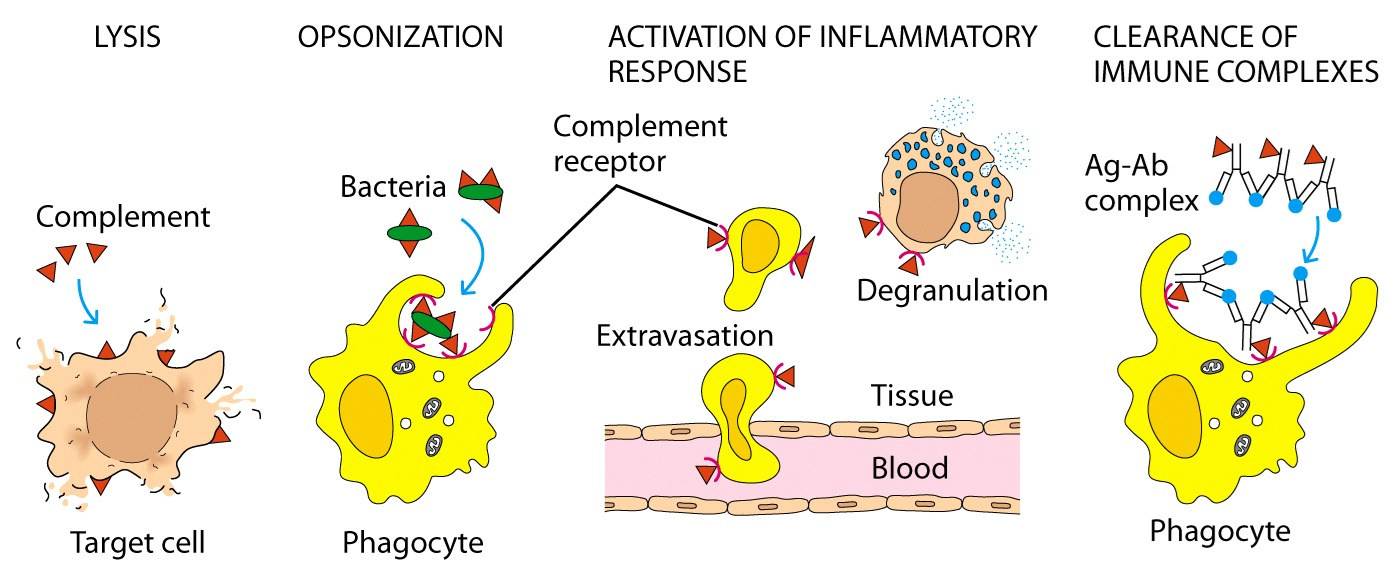
importance of complement
importance of MC which causes lysis
opsonisation —> phagocytes have receptors for opsonised bacteria and so enhances recognition
complement receptors are also on the neurophils which helps with extravasion
might cause degradation of mast cells
also potent in clearance of the immune complex
can recognise apoptitic cells
forms a bridge between innate and adaptive immune system
neutrophils
mechanism that facilitate inflammation
short lived - a few hours
produced in the bone marrow
attracted by chemokines IL8 and C5a
can detect and phagocytose pathogens
effector mechanisms to kill pathogen
results in pus
macophage/mast cells produce histamine which can signal to cells in the blood
cells in the blood when signalled to will extravate into the tissues
leukocyte extravasion
chemoattraction of leukocytes —> they tether and start rolling on the endothelium which slows them down
mediated through selectin interations
similar in lymphocytes
the final step is migration between tight junctions
MECHANISM
tethering and rolling
siayl lewis X on neutrophils binds to E&P selectins on endothelial cells
the integrin LFA1 binds to E and P selectins on endothelial cells
slowing down and transient adhesion of neutrophils
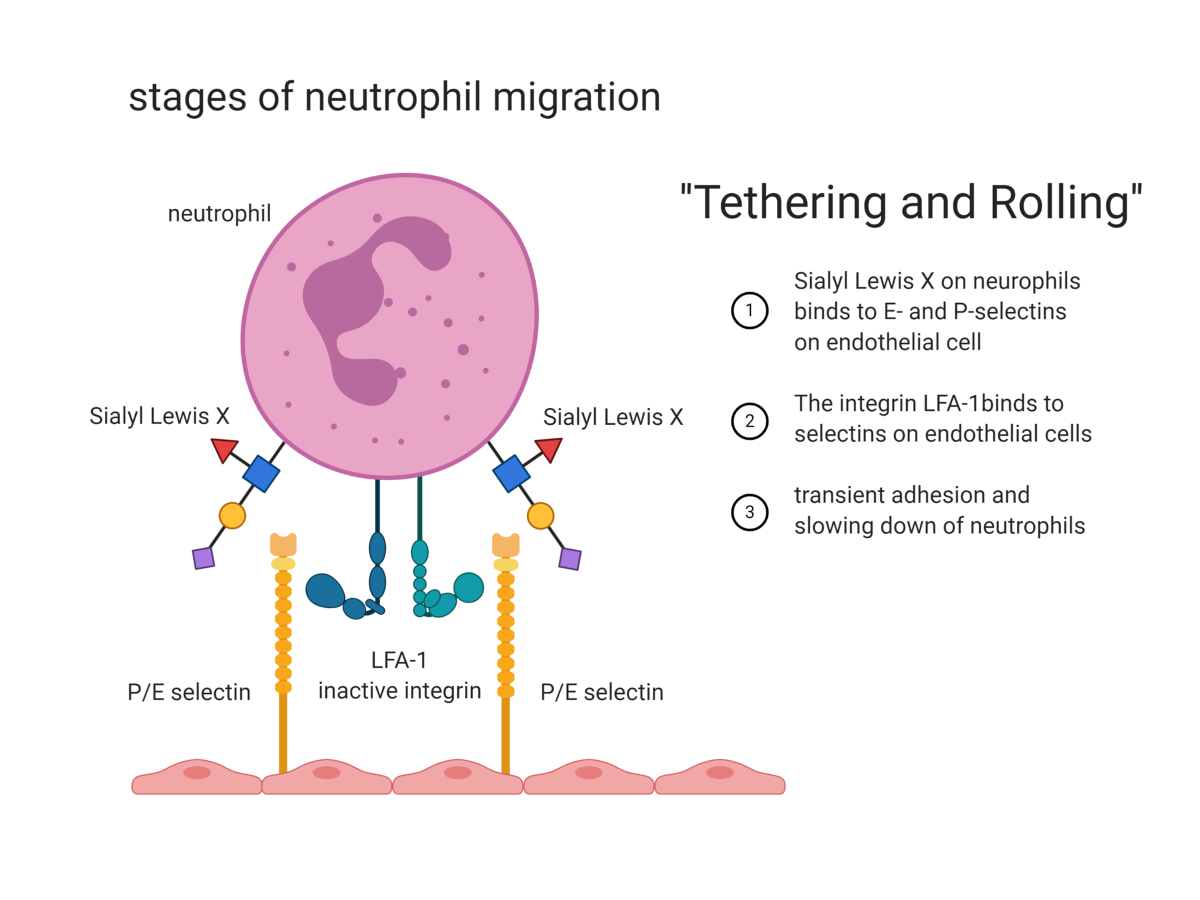
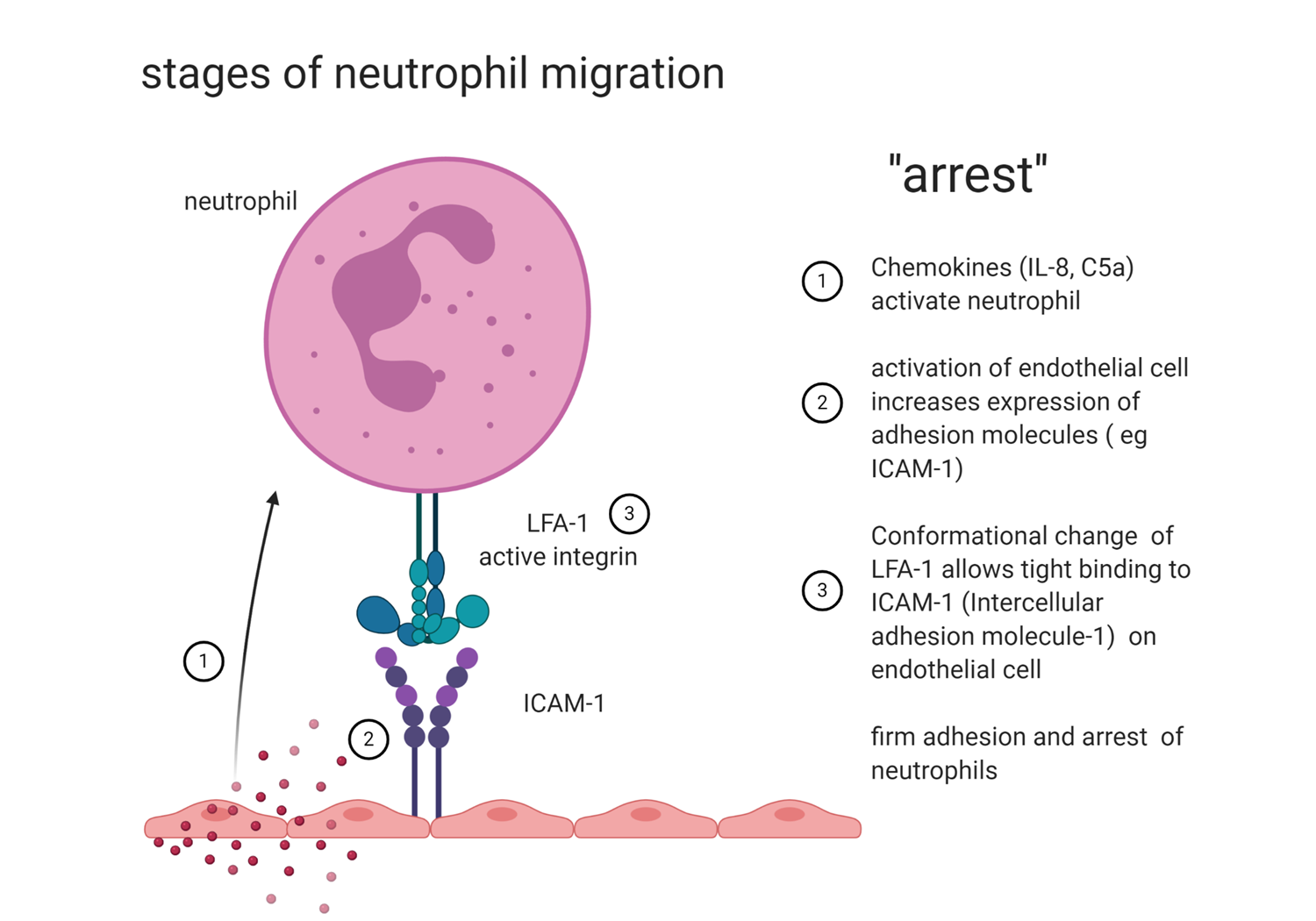
ARREST (driven by chemokines)
cchemokines activate neutrophils
activation of endothelial cells increases the expression of adhesion molecules
a conformational change of LFA1 allows tight binding to ICAM1 on endothelial cells
firm adhesion and arrest of neutrophil
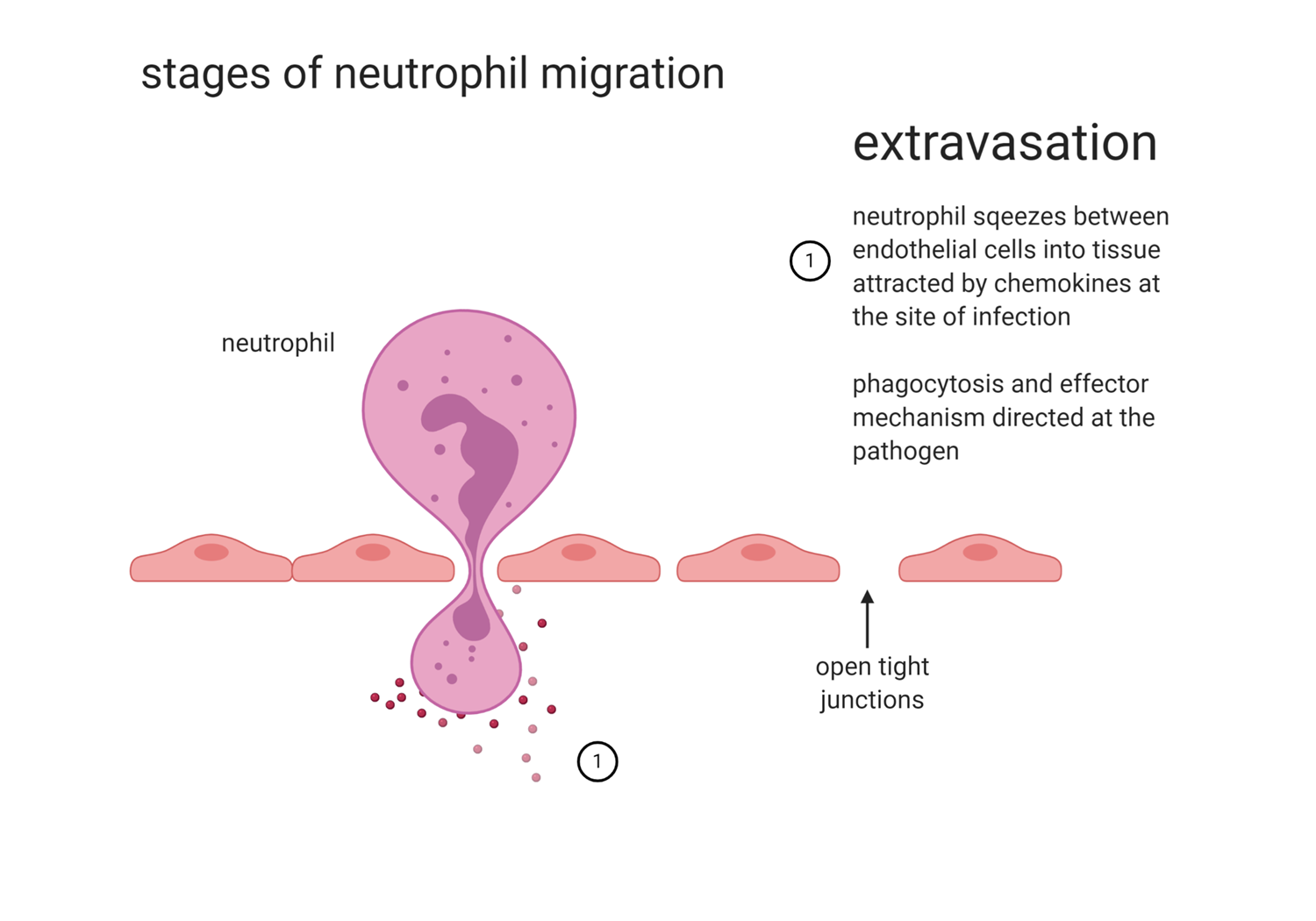
EXTRAVASION
neutrophil squeezes between endothelial cells into tissues attracted by chemokines at the site of attraction
phagocytosis and effector mechanisms directed at the pathogen (still dependent on chemokines)
t cells, monocytes, dendritic cells all help in recruitment of chemokines
chemokines expressed on immune cells cal allow viruses to enter the cells
macrophages
recognise particles, bacteria, dyes etc
many diff types with homeostatic functions to ensure normal physiology
liver - kupffer cell
brain - microglia
bone - osteoblast
lung - alveolarmacrophage
monocyte derived macrophages important in immune response
activated macrophage has different function to homeostatic macrophage
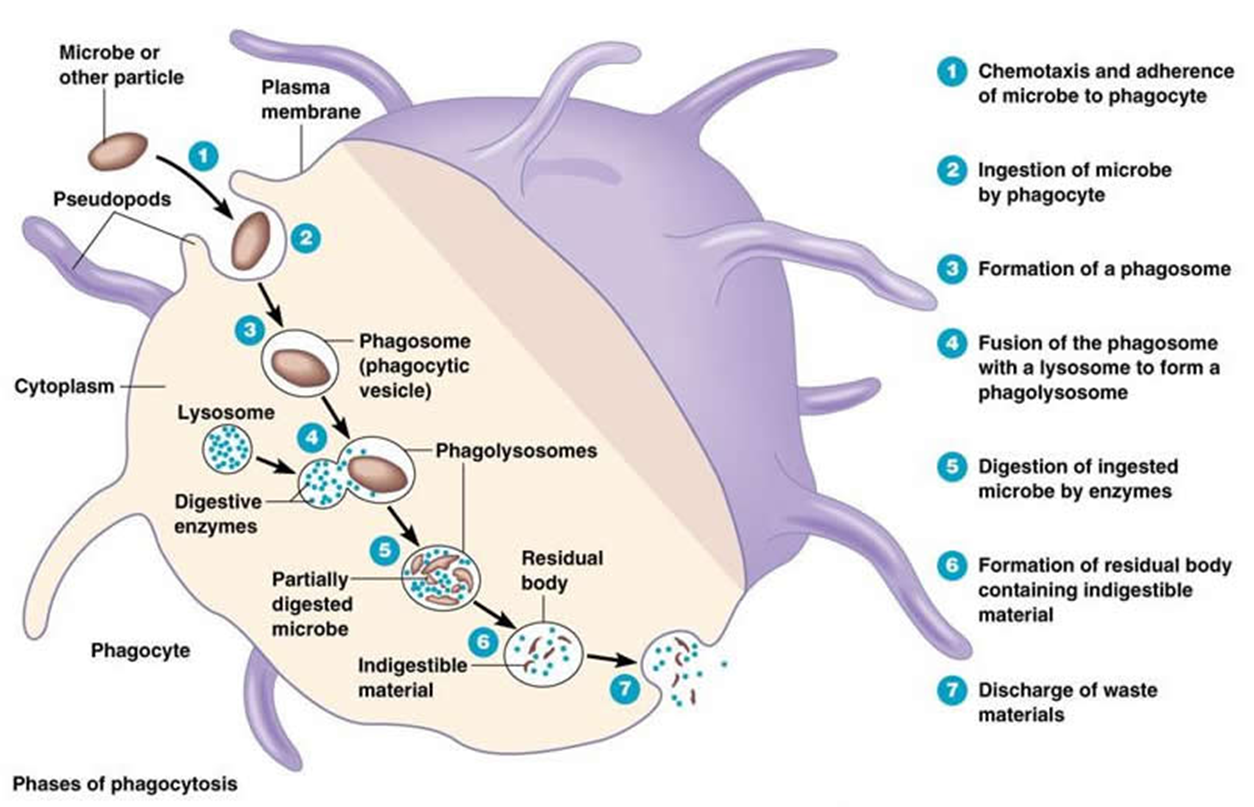
macrophage mechanism of uptake
recognised by pseudopods and taken up
forms a phagosome which fuses with the lysososme
the phagolysosome partially digests the microbe
sometimes the antigens are presented on the surface which links to the adaptive immune system
till like receptors, mannose, glucan receptors, scavenger receptors all halp with the uptake of cells
there are also different methods of pathogen destruction
acidification (3.5 - 4.0 pH)
toxic O2 derived products which are highly reactive oxygen radicals
antimicrobial peptides
enymes - lysozmyes
competitors