cellular organization of the nervous system
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
principal cells of the nervous system
neuron
glia = “glue”
outnumber neurons 10:1
glial cells of CNS
microglia
astrocytes
oligodendrocytes
ependymal cells
microglia
smallest glial cell
immune response in brain
destroy microorganisms
clear debris
promote tissue repair
scavenge for damaged neurons and protein plaques
clear old synapses —> get rid of what’s not useful
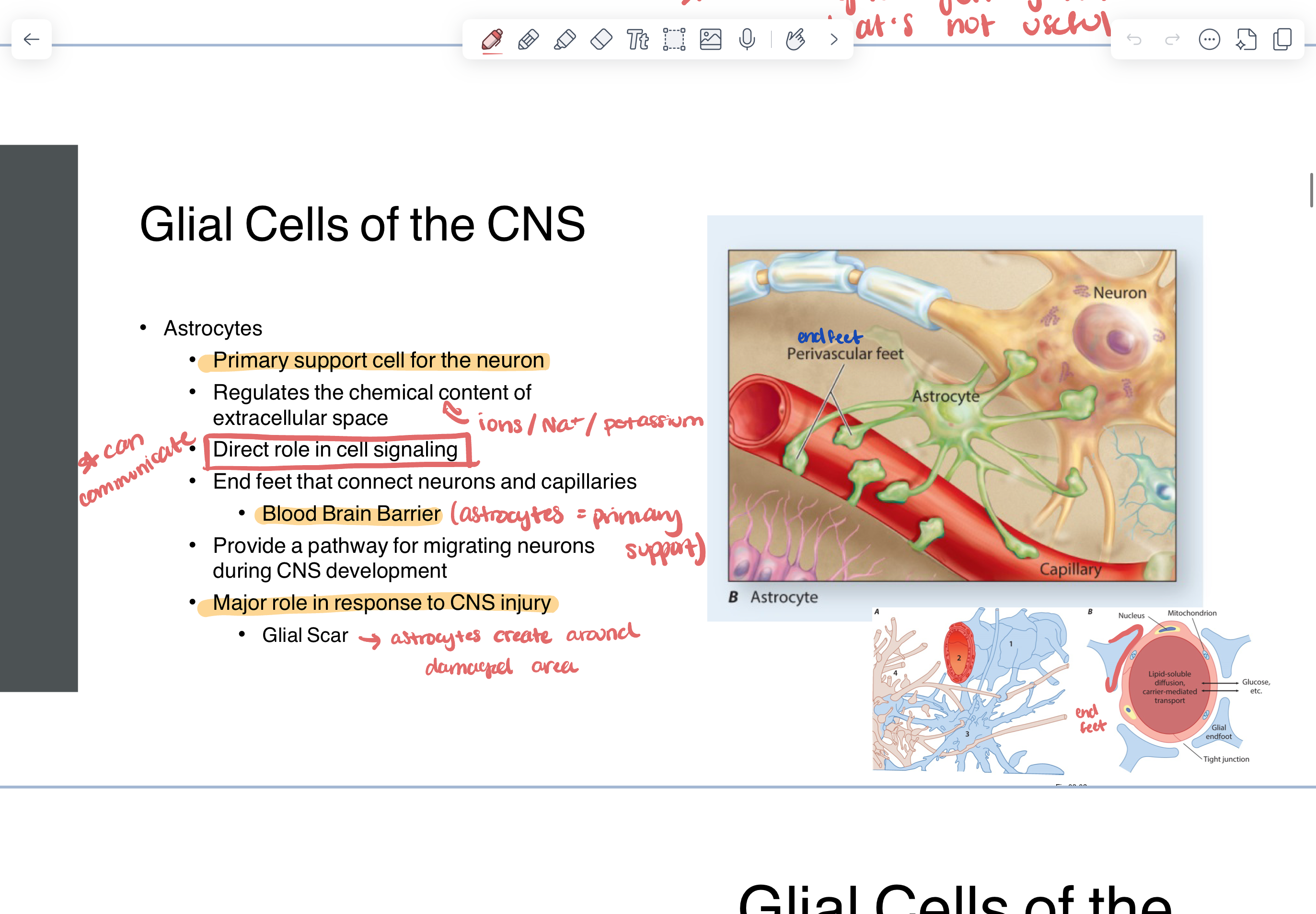
astrocytes
primary support cell for neuron
regulates the chemical content of extracellular space (ions, Na+, K+)
direct role in cell signaling (can communicate)
end feet that connect neurons and capillaries
blood brain barrier
major role in response to CNS injury
glial scar —> astrocytes create around damaged area
oligodendrocytes
myelin producing
proteins and fats that surround neurons
provide metabolic support to axon
speed communication through axon
ependymal cells
line ventricles to form the choroid plexus
choroid plexus - produces and circulates CSF
regulate ionic concentration
glial cells of PNS
Schwann cells
satellite cells
Schwann cells
myelinating glia of PNS
provide metabolic support and speed communication through axon
connective tissue scaffold
development
repair
satellite cells
found primarily in ganglia - structural support for nodes in ganglia
provide nutrients and control environment around neuron
collection of neural cell bodies in the PNS
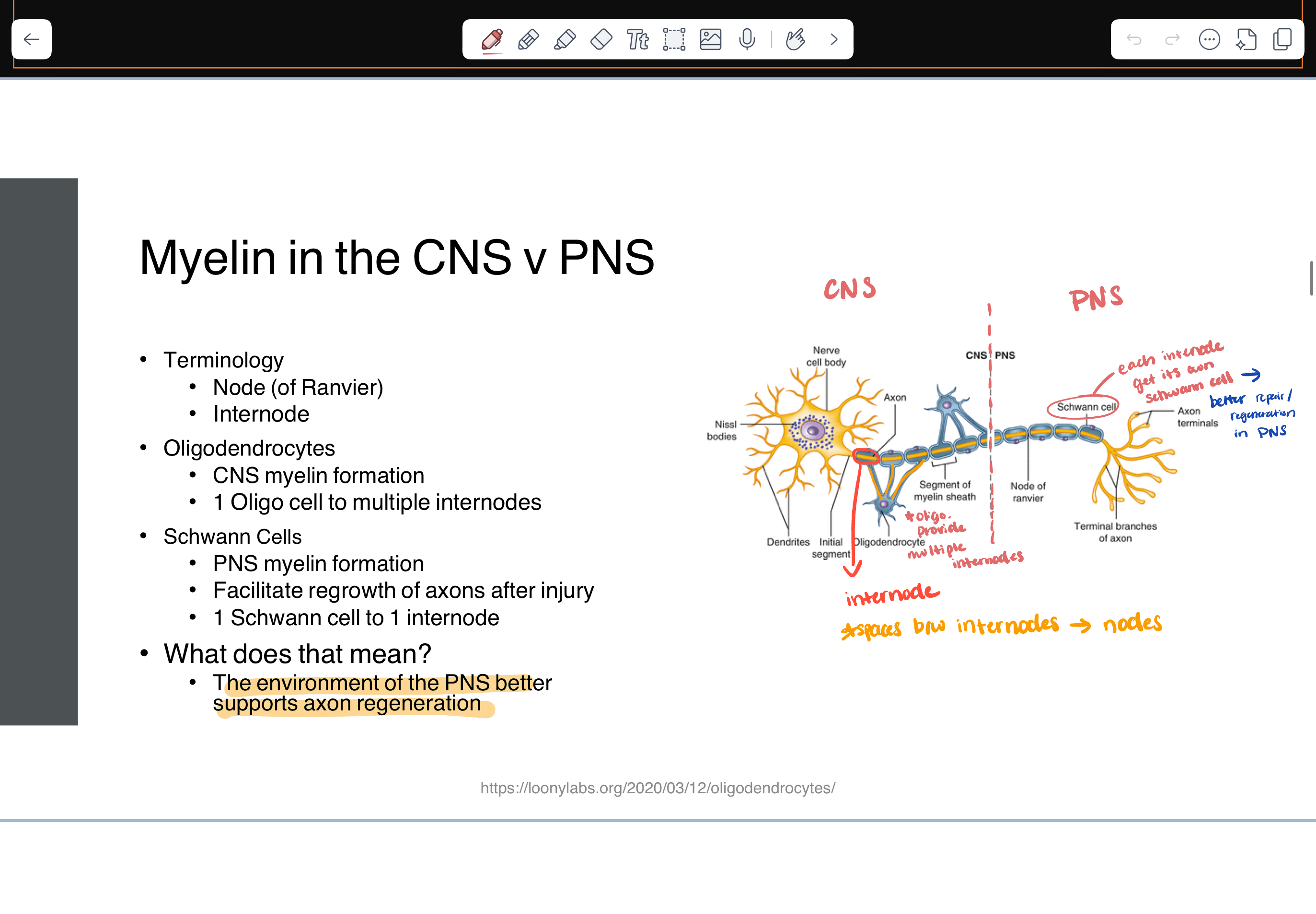
myelin in CNS vs PNS
terminology
node of ranvier - spaces between internodes
internode
oligodendrocytes
CNS myelin formation
1 oligo cell to multiple internodes
Schwann cells
PNS
1 Schwann cell to 1 internode
the PNS better supports axon regeneration
multiple sclerosis
CNS demyelinating diseases
chronic autoimmune disease
inflammation
demyelination
gliosis —> damage to glia
neuronal loss
major processes
inflammation resulting in plaques and injury to BBB
neurodegeneration
axons, neurons, synapses
myelin regulates axon —> fairly damaging
guillain barre syndrome
PNS
immune mediated neuropathy
destruction of Schwann cells and neurons
flaccid paralysis (proximal to distal)
life threatening (respiratory function)
neurons structure & function
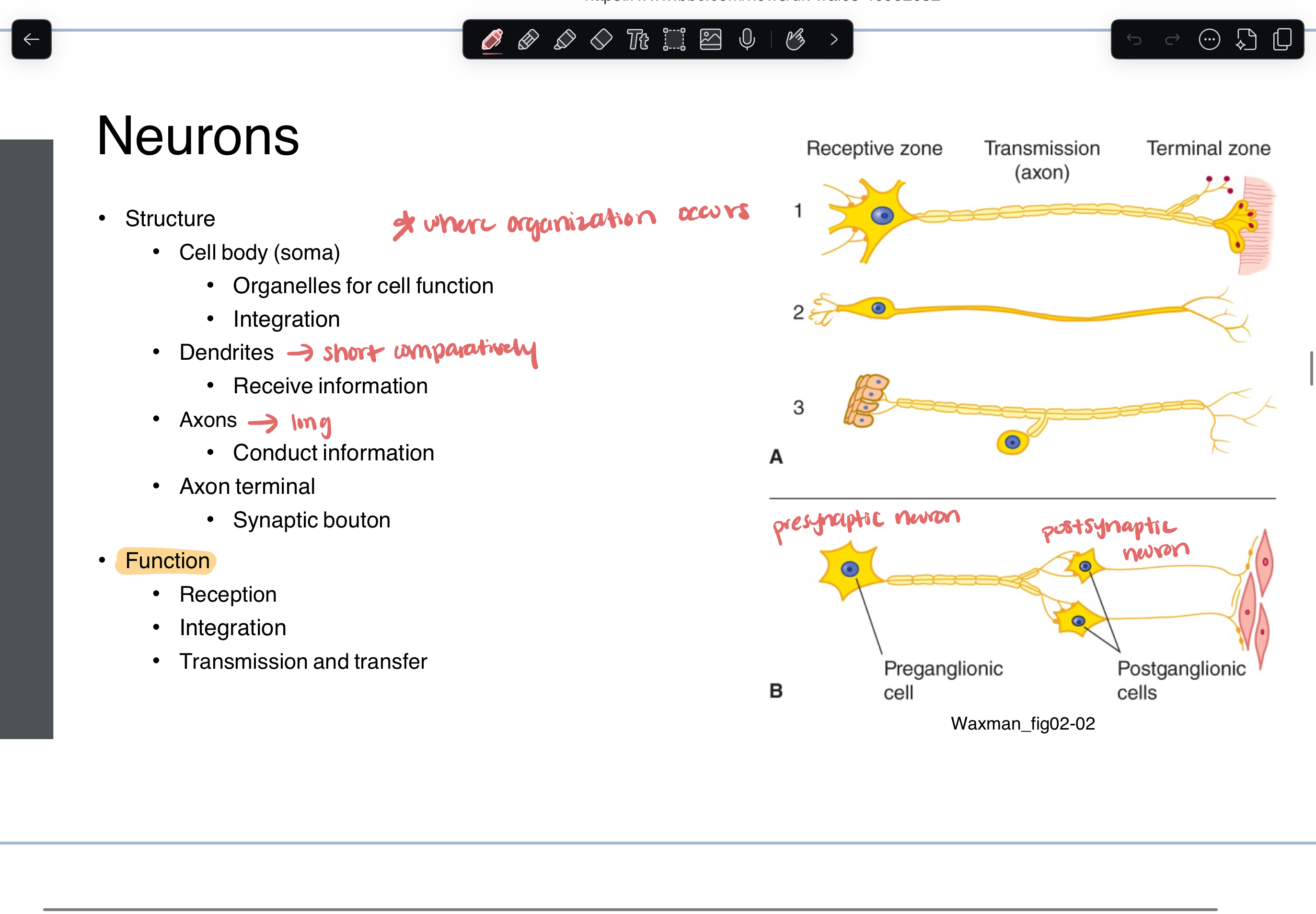
structure
cell body (integration)
dendrites
short
receive information
axons
long
conduct information
axon terminal
synaptic bouton
function
reception
integration
transmission and transfer
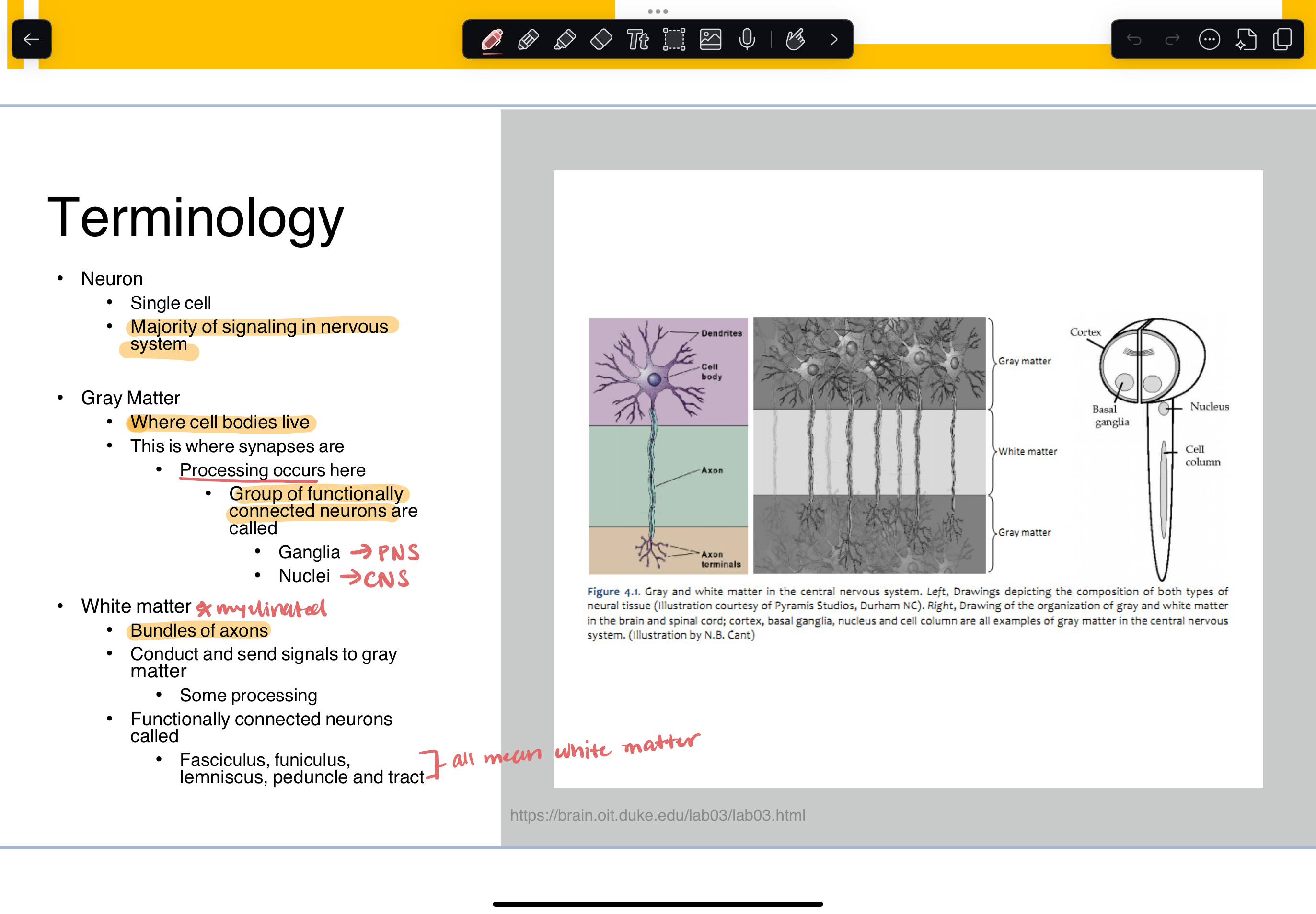
gray matter vs white matter
gray matter
where cell bodies live
this is where synapses are (processing occurs here)
group of functionally connected neurons are called
ganglia (PNS)
nuclei (CNS)
white matter
myelinated
bundles of axons
conduct and send signals to gray matter
functionally connected neurons called:
fasciculus, funiculus, lemniscus, peduncle and tract (all mean white matter)
electrical and chemical signals
electrical = action potential
chemical = neurotransmitter
dendrites and cell bodies receive info
transmitted down axon as electrical signal
at synapse, electrical signals cause release of chemical messenger into the synapse
chemical messenger is received by post synaptic dendrite and converted into electrical signals
resting
membrane potential
the difference in the electrical charges inside and outside the cell create an electric potential
-65mV
steady state, no net flow of ions
metabolic energy is expended to maintain this gradient (difference)
Na+/K+ pump
ions cross membrane through channels
modality gated - photoreceptors, mechanoreceptors, thermoreceptors
ligand gated
direct:
NT binds and directly opens the channel
faster but effects are local
indirect
NT binds and through a second messenger system that will bind and open the channel
G protein coupled receptors are most common
slower but more widespread effects
voltage gated
change in electric potential
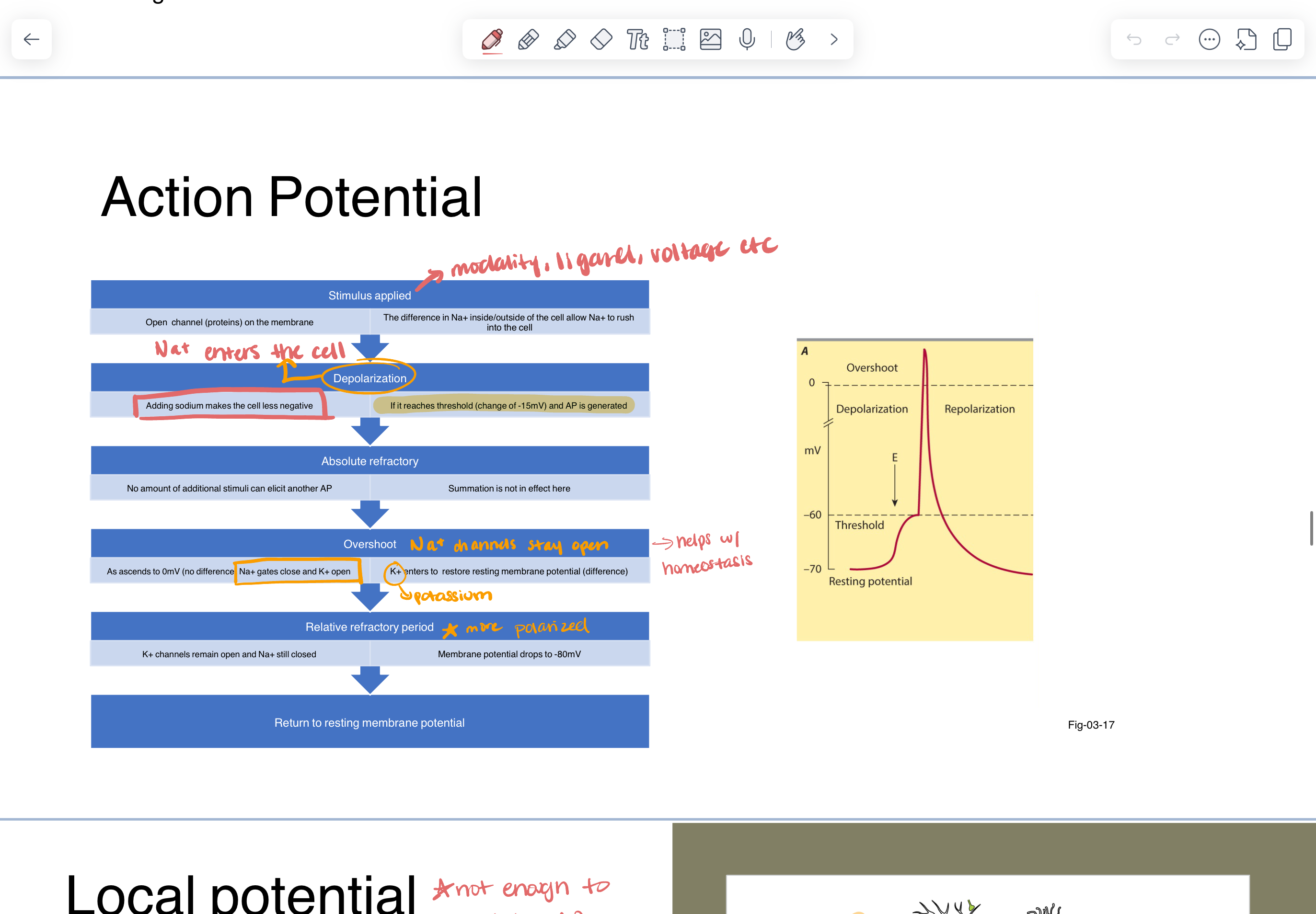
action potential
momentary change in the electrical gradient across cellular membrane
creates an electrical signal that can move down the axon —> propagate
“all or nothing” —> if the change in the membrane is sufficient an action potential will be generated
action potential coded
stimulus applied —>
difference in Na+ inside/outside cell causes Na+ to rush into cell —> this makes inside of cell less negative (depolarization)
if threshold change is -15mV (an AP is generated)
absolute refractory
no amount of additional stimuli can elicit another AP
as potential ascends to 0mV —> Na+ gates close and K+ open —> K+ enters to restore resting membrane potential
relative refractory period
K+ channels stay open
more polarized
return to RMP
local potential
not enough to stimulate AP
small changes in membrane potential
do not reach threshold
can be summated
spatial - multiple axons acting on another axon (same geographic area)
temporal - over the course of time

changes in potential can make it…
MORE likely for AP to fire
depolarized
excitatory post synaptic potential (EPSP) —> Na+
LESS likely for AP
hyperpolarized
inhibitory post synaptic potential (IPSP) —> chlorine (Cl-) channels open instead of Na+
structural adaptations to speed up AP
myelin
nodes of ranvier contain dense cluster of Na+ channels
saltatory conduction —> myelin allows signal to jump from node to node
diameter —> reduces resistance to charge (fatter axons = fast)
synaptic terminal events
AP arrives at axon terminal —> activates voltage gates (Ca+ channels)
Ca+ enters the cell
Ca+ activates vesicle binding
brings vesicles filled with neurotransmitters to membrane
NT released into synaptic cleft
NT diffuse across membrane
NT bind to receptors in post synaptic membrane
if ESPS —> increase Na+
if ISPS —> increase Cl-
removal of NT from synapse
broken down with enzymes
simple diffusion
reuptake into presynaptic terminal
anterograde
NT from cell body —> towards axon terminal
kinesin
retrograde
NT in axon terminal —> towards cell body
dynein
botulinum toxin
blocks action at the neuromuscular junction
prevents release of acetylcholine (ACh) from the LMN to muscle cell
ACh is primary NT at NMJ
botox to brachialis —> block release of NT from musculocutaneous nerve
treatment for spasticity
paralyzes face muscles - blocks activity of NT binding to synaptic membrane
retrograde transport is a mechanism for…
pathogen entry
herpes encephalitis
rabies
tetanus
diseases at NMJ
primary symptom = weakness
myasthenia gravis
disease of receptor
autoimmune disease that attacks ACh receptors
treated with drug that blocks Ach esterase (role is to break down ACh)
Lambert Eaton Syndrome
autoimmune disease that attacks Ca+ channels
results in reduced ACh release into MJ = weaker muscle contractions
if ACh can hang out in cleft longer —> better muscle contraction
wallerian degeneration
when the axon is injured, glial cells remove the damaged areas
axon stump recedes in preparation for signal to regenerate
cell body prepares for regrowth
chromatolysis
nervous system response to injury
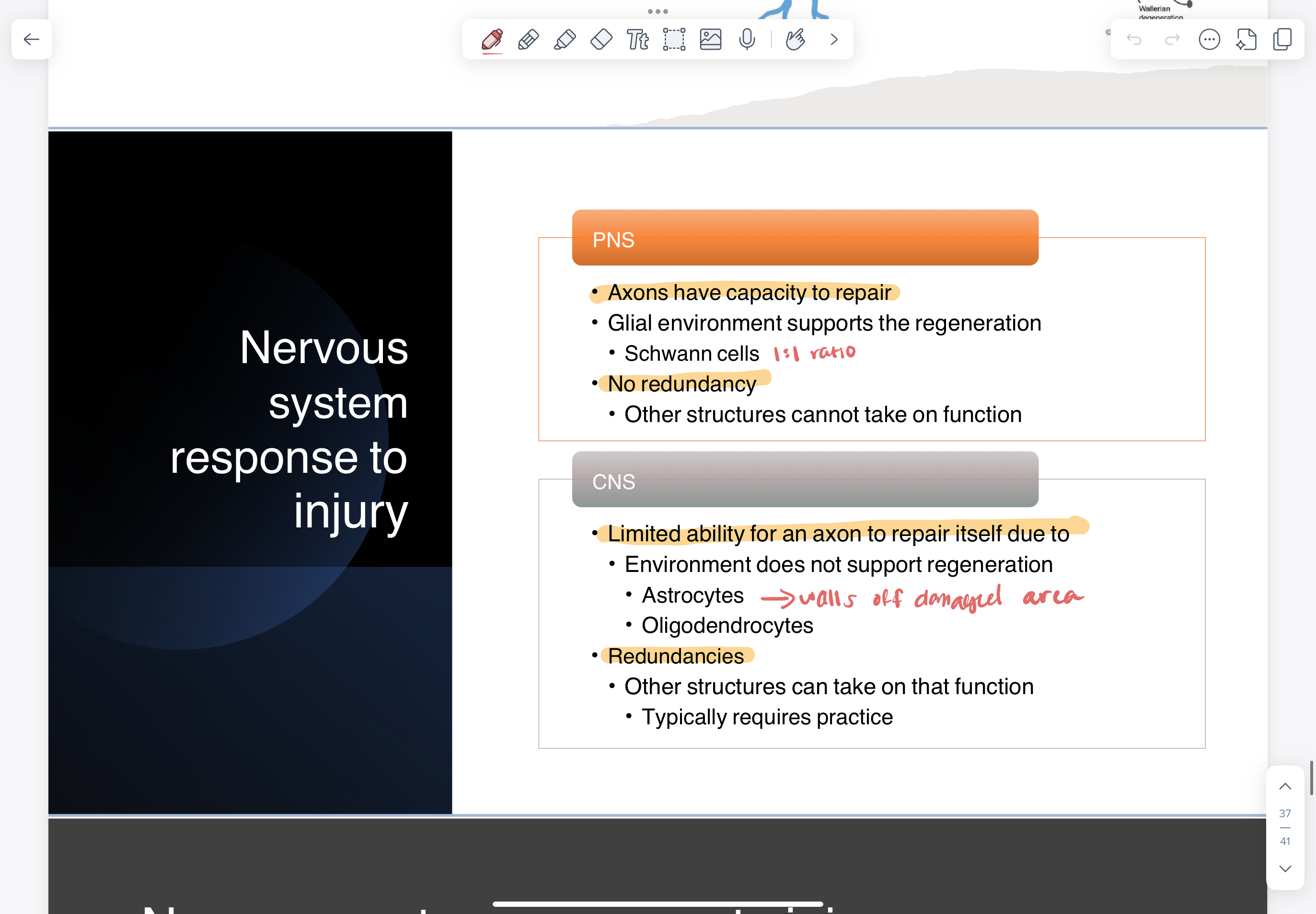
PNS response to injury
recovery depends on the extent of damage
if some connective tissue remains
Schwann cells can scaffold and direct the regeneration to the target recovery
1:1 ratio —> produce substances to support axon growth
if axon completely severed
no scaffolding for regeneration
recovery is not expected without surgery
CNS response to injury
very dependent on body’s practice
after injury —> damaged area is walled off
microglia clear debris
astrocytes create physical wall around area
glial scar —> secretes substances that prevent axons from moving past
oligodendrocytes
many oligo:node so don’t have scaffolding abilities of PNS
secrete protein Nogo that limits sprouting —> prevents regeneration
this protein is useful in development of circuits to maintain order of tracts
not helpful after injury
the CNS can reorganize —> plasticity
uninjured axons and dendrites can extend to other territories
take on damaged axon’s roles